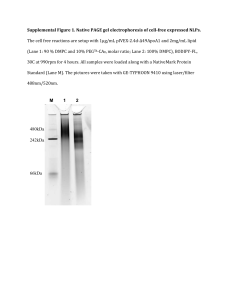
1176 Supporting information
advertisement

Supporting information The Dehydration Dynamics of Model Cell Membrane Induced by Cholesterol Analogue 6-Ketocholestanol Investigated Using Sum Frequency Generation Vibrational Spectroscopy Sulan Ma,1 Kangzhen Tian1,2, Shuji Ye,1,2* 1 Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemical Physics, University of Science and Technology of China, Hefei, Anhui, P.R.China 230026; 2Synergetic Innovation Center of Quantum Information & Quantum Physics, University of Science and Technology of China, Hefei, Anhui 230026, China 1. Fitting of SFG-VS Signal As described in detail elsewhere, the intensity of the SFG light is related to the square of ( 2) the sample's second-order nonlinear susceptibility eff , and the intensity of the two input fields I ( IR ) and I ( vis ) , see eq. (1), which vanishes when a material has inversion symmetry [1-6]. 2 ( 2) I ( SFG ) eff I 1 ( vis ) I 2 ( IR ) (S1) where SFG IR vis . As the IR beam frequency is tuned over the vibrational resonance of surface/interface molecules, the effective surface nonlinear susceptibility R( 2) can be ( 2) enhanced. The frequency dependence of eff is described by ( 2) ( 2) eff ( ) NR A (S2) i where A , , and are the strength, resonant frequency, and damping coefficient of the vibrational mode(), respectively. A could be either positive or negative depending on the S1 phase of the vibrational mode. The plot of SFG signal vs. the IR input frequency shows a polarized vibrational spectrum of the molecules at surface or interface. A , , and can be extracted by fitting the spectrum. 2. Figure S1-Figure S6 a) b) c) d) Figure S1. Molecular structure of a) 6-ketocholestanol; b) DMPC; c) DMPG d) DMEPC. S2 A Surface pressure (mN/m) Surface pressure (mN/m) 50 KCL KBr KNO3 40 B DMPC:6-KC=12: 20 DMPC:6-KC=12: 15 DMPC:6-KC=12: 10 DMPC:6-KC=12: 5 DMPC:6-KC=12: 1 DMPC 50 Water K2SO4 KI KSCN 30 20 40 30 20 10 10 0 0.2 0.4 0.6 0.8 2 1.0 0 0.2 1.2 Mean molecular area(nm /molecule) 0.4 0.6 0.8 1.0 2 1.2 1.4 Mean molecular area(nm /molecule) Figure S2. The surface pressure-area (-A) isotherms of A)DMPC at air/ different salt solution (100 mM); B) The mixture of DMPC and 6-KC with different DMPC/6-KC ratios Intensity at air/water interface. 1.2 0.8 0.4 0.0 1.0 0.5 0.0 1.2 0.6 0.0 0.8 0.4 0.0 0.4 0.0 a) b) c) d) e) f) 0.3 0.0 0.2 g) 0.0 0 200 400 600 Time(min) Figure S3 Time dependence of ssp SFG intensity at different wave numbers collected from S3 d-DMPC bilayer/water interface at different time after adding 3L 6- ketocholestanol solution at t = 0 min: a). 3000 cm-1; b). 3100 cm-1; c). 3200 cm-1; d). 3300 cm-1; e). 3400 cm-1; f). 3500 cm-1; g). 3600 cm-1. SFG Intensity 2 a) 0 4 2 0 6 3 0 4 2 0 2 0 2 0 1 0 b) c) d) e) f) g) 0 100 200 300 400 500 600 Time(min) Figure S4 Time dependence of the ssp SFG intensity at different wave numbers collected from DMEPC bilayer/water interface at different time after adding 10 L 6-ketocholestanol solution: a). 3000 cm-1; b). 3100 cm-1; c). 3200 cm-1; d). 3300 cm-1; e). 3400 cm-1; f). 3500 cm-1; g). 3600 cm-1. S4 0.4 SFG intensity 1.2 0.3 0.2 ssp 0.1 (2) psp SFG Intensity A B 0.9 0.6 0.3 0.0 1.2 C 0.9 0.6 0.3 0.0 0.0 0 50 100 150 0 200 Time(min) 40 80 120 160 Time(min) Figure S5. (A) Time dependent psp spectra intensity at 2935 cm-1 after injecting 6-ketocholestanol into DMEPC bilayer at t=0; (B) Time dependent ssp intensity at 1710 cm-1; (C) Time dependent fitting amplitude of 1710 cm-1 peak in the ssp spectra. 0.15 a 0.10 0.05 SFG Intensity 0.00 b 0.10 0.05 0.00 0.10 c 0.05 0.00 0.10 d 0.05 0.00 2800 2900 3000 3100 -1 Wavenumber(cm ) Figure S6. The psp spectra of 6-ketocholestanol after injecting 10 L 6-ketocholestanol S5 solution into the mixed DMPC/DMPG bilayer/water subphase. a) DMPC/DMPG=75/25; b) DMPC/DMPG=50/50; b) DMPC/DMPG=25/75; b) DMPC/DMPG=0/100. Reference 1. Shen YR. The Principles of Nonlinear Optics; Wiley: New York, 1984. 2. Castellana ET, Cremer PS, Solid Supported Lipid Bilayers: From Biophysical Studies to Sensor Design. Surf Sci Rep, 2006, 61, 429-444. 3. Lambert A, Davies P, Neivandt D. Implementing the Theory of Sum Frequency Generation Vibrational Spectroscopy: A Tutorial Review. Appl Spectrosc Rev, 2005, 40: 103-145. 4. Gopalakrishnan S, Liu D, Allen HC, Kuo M, Shultz MJ. Vibrational Spectroscopic Studies of Aqueous Interfaces: Salts, Acids, Bases, and Nanodrops. Chem Rev, 2006, 106: 1155-1175. 5. Wang HF, Gan W, Lu R, Rao Y, Wu BH. Quantitative Spectral and Orientational Analysis in Surface Sum Frequency Generation Vibrational Spectroscopy (SFG-VS). Int Rev Phys Chem. 2005, 24: 191-256. 6. Ye SJ, Wei F, Li HC, Tian KZ, Luo Y. Structure and Orientation of Interfacial Proteins Determined by Sum Frequency Generation Vibrational Spectroscopy: Method and Application. Adv Protein Chem Struct Biol, 2013, 93: 213-255. S6