PHYS 1401 General College Physics I
advertisement

PHYS 1401 General College Physics I
(43172 - MTWR 5:30-7:50 PM)
Home Work 12:
(pages 366-369)
1, 5, 9, 13, 15, 29, 35, 43, 57, 67
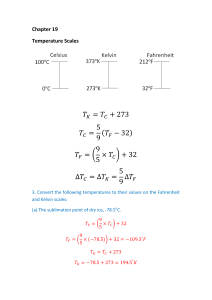
1.
The difference between these two averages, expressed in Fahrenheit degrees, is
98.6 F 98.2 F 0.4 F
Since 1 C° is equal to
9
F°, we can make the following conversion
5
1 C
(0.4 F)
0.2 C
(9/5) F
5.
The temperature of –273.15 °C is 273.15 Celsius degrees below the ice point of 0
°C. This number of Celsius degrees corresponds to
(9/5) F
(273.15 C)
491.67 F
1 C
Subtracting 491.67 F° from the ice point of 32.00 °F on the Fahrenheit scale gives
a Fahrenheit temperature of –459.67 F
9.
Using the value for the coefficient of thermal expansion of steel given in Table
12.1, we find that the linear expansion of the aircraft carrier is
L = L0 T = (12 10–6 C–1)(370 m)(21 °C 2.0 °C) 0.084 m
(12.2)
13.
The value for the coefficient of thermal expansion of steel is given in Table 12.1.
The relation, L = L0T, written in terms of the diameter d of the rod, is
d
0.0026 cm
T
110 C
(12.2)
6
d0
12 10 C 1 2.0026 cm
15.
Assuming that the rod expands linearly with heat, we first calculate the quantity
L/ T using the data given in the problem.
L
8.47 10–4 m
1.13 10 –5 m/C
T 100.0 C – 25.0 C
Therefore, when the rod is cooled from 25.0 °C, it will shrink by
L (1.13 10 –5 m/C ) T
(1.13 10 –5 m/C ) (0.00 C – 25.0 C) –2.82 10 –4 m
29.
According to Equation 12.3, V VT . Taking the coefficient of volumetric
expansion for water from Table 12.1, we find that
Vair
V T
3.7 10–3 (C)–1
air 0
air
18
Vwater water V0 T water 207 10 –6 (C)–1
35.
In order to keep the water from expanding as its temperature increases from 15 to
25 °C, the atmospheric pressure must be increased to compress the water as it tries to
expand. The magnitude of the pressure change P needed to compress a substance by
an amount V is, according to Equation 10.20, P B(V / V0 ) . The ratio V / V0 is,
according to Equation 12.3, V / V0 T . Combining these two equations yields
P B T
SOLUTION Taking the value for the coefficient of volumetric expansion for
water from Table 12.1, we find that the change in atmospheric pressure that is required to
keep the water from expanding is
1
P (2.2 109 N/m 2 ) 207 106 C (25 C 15 C)
1 atm
4.6 106 Pa
45 atm
5
1.0110 Pa
43. The metabolic processes occurring in the person’s body produce the heat that is added
to the water. As a result, the temperature of the water increases. The heat Q that
must be supplied to increase the temperature of a substance of mass m by an amount
T is given by Equation 12.4 as Q = cmT, where c is the specific heat capacity. The
increase T in temperature is the final higher temperature Tf minus the initial lower
temperature T0. Hence, we will solve Equation 12.4 for the desired final temperature.
SOLUTION From Equation 12.4, we have
Q cmT cm Tf T0
Solving for the final temperature, noting that the heat is Q = (3.0 × 105 J/h)(0.50 h)
and taking the specific heat capacity of water from Table 12.2, we obtain
3.0 105 J/h 0.50 h
Q
Tf T0
21.00 C
21.03 C
cm
4186 J/ kg C 1.2 103 kg
57.
The heat required is Q = mLf + cmT, where m = V. See Table 12.2 for the
specific heat c and Table 12.3 for the latent heat Lf. Thus,
Q = VLf + cVT
Q = (917 kg/m3)(4.50 10–4 m)(1.25 m2){33.5 104 J/kg
+ [2.00 103 J/(kgC°)](12.0 C°)} 1.85 10 5 J
67.
From inspection of the graph that accompanies this problem, a pressure of
3.5 106 Pa corresponds to a temperature of 0 °C. Therefore, liquid carbon dioxide
exists in equilibrium with its vapor phase at 0 C when the vapor pressure is
3.5 106 Pa .