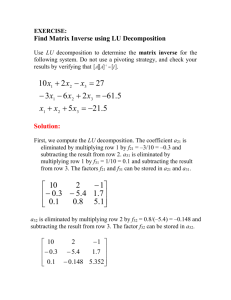
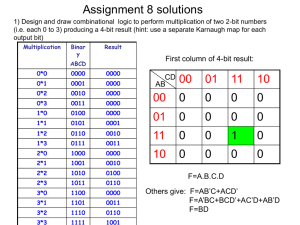
Senior Design Paper
advertisement

Letter of Transmittal GLYDE Company 1000 Johnson Dr. Port Arthur, TX 77640 April 24, 2006 Dr. George Rowell Drexel University, Department of Chemical & Biological Engineering 3141 Chestnut St. Philadelphia, PA 19104 Dr. Rowell: Enclosed you will find the feasibility study for the production of ethylene glycol by means of direct oxidation of ethylene over a silver based catalyst followed by hydration of ethylene oxide over a solid phase ion exchange resin. The total capital investment is estimated at $124 million. With an Internal Rate of Return after a 16 year lifespan of 30%, this plant will exceed the hurdle rate of 13%. The break even period for this project is 2 years which shows a highly feasible project. Further development for this plant includes reducing utilities and recovering and purifying carbon dioxide byproduct for sale. Our group greatly appreciates your consultation and guidance in this project. Sincerely, Suroor Manzoor Chong McLaren Nick Mitchell Timre Segear Letter of Transmittal GLYDE Company 1000 Johnson Dr. Port Arthur, TX 77640 April 24, 2006 Mr. Stevon G. Schon, P.E. Archema Chemicals Inc., 900 First Avenue King of Prussia, PA 19406 Mr. Schon: Enclosed you will find the feasibility study for the production of ethylene glycol by means of direct oxidation of ethylene over a silver based catalyst followed by hydration of ethylene oxide over a solid phase ion exchange resin. The total capital investment is estimated at $124 million. With an Internal Rate of Return after a 16 year lifespan of 30%, this plant will exceed the hurdle rate of 13%. The break even period for this project is 2 years which shows a highly feasible project. Further development for this plant includes reducing utilities and recovering and purifying carbon dioxide byproduct for sale. Our group greatly appreciates your consultation and guidance in this project. Sincerely, Suroor Manzoor Chong McLaren Nick Mitchell Timre Segear Production of Ethylene Glycol Suroor Manzoor Chong McLaren Nick Mitchell Timre Segear Technical Advisor: Dr. George Rowell Industrial Advisor: Mr. Stevon Schon, P.E. April 24, 2006 Department of Chemical and Biological Engineering Drexel University Philadelphia, PA 19104 Production of Ethylene Glycol Suroor Manzoor Chong McLaren Nick Mitchell Timre Segear Technical Advisor: Dr. George Rowell Industrial Advisor: Mr. Stevon Schon, P.E. April 24, 2006 Department of Chemical and Biological Engineering Drexel University Philadelphia, PA 19104 Abstract Currently more than 65% of the ethylene oxide produced in industry is used in the production of ethylene glycol. GLYDE will convert all of the ethylene oxide produced to ethylene glycol to maximize profits, minimize inherently dangerous inventories, and increase safety in handling and transporting products. This plant will be located in Port Arthur, Texas, within a few miles of an existing ethylene pipeline and near major ports to cheaply transport both raw materials and final products. Based on the average production rates of the major suppliers, GLYDE’s plant is designed to produce approximately 890 million pounds per year of ethylene glycol based on 8,100 hours of operation per year. This will allow GLYDE to occupy 3.2% of the US market for ethylene glycol upon entry, and up to 13% at full capacity within three years. As there has been no significant net addition of new capacity in the industry since 2001, there is a measurable shortfall in the ethylene glycol supply. Overall, the ethylene glycol demand has increased by 6-7% per year since 2001 and is expected to increase at this rate through 2010 (20). In the first year GLYDE’s plant is expected to run for half a year at 50% capacity. The second year is expected to run for nine months at 67% capacity, and the plant will run at full capacity there after. The direct oxidation technology is the dominant process to produce ethylene oxide commercially today. It utilizes the catalytic oxidation of ethylene with pure oxygen over a silver based catalyst to yield ethylene oxide. We have chosen to use pure oxygen as opposed to air as an oxidizer to reduce the amount of inerts in the system, which reduces the total purge necessary and minimizes operating costs. Also pure oxygen leads to higher selectivity to ethylene oxide. The average selectivity ranges from 65-75% for the air-based process compared to 85-90% for the oxygen-based process. Ethylene glycol is commercially produced by the hydrolysis of ethylene oxide with or without a catalyst. As of right now, catalytic hydration of ethylene oxide using an ion exchange resin is a new technology in industry and is gaining popularity. A highly selective solid phase ion exchange resin as a catalyst allows us to convert 98% of the ethylene oxide to monoethylene glycol with the other 2% converted to diethylene glycol. The catalyst also allows for a reduction in the ratio of water to ethylene oxide and the temperature in the reactor which in turn lowers operating costs. Executive Summary The scope of this project is to determine the feasibility of the production of ethylene glycol through hydration of ethylene oxide which is produced by the direct oxidation of ethylene. We have determined that the raw material cost of ethylene is by far the leading factor in the cost of production of ethylene glycol. The raw materials alone contribute to 17.6 c/lb of the total 21 c/lb for the cost of ethylene glycol production. Because of this, the major process decisions were all geared toward maximizing selectivity to our desired products, and minimizing the total amount of wasted ethylene. We have chosen to use pure oxygen as opposed to air in the oxidation of ethylene to reduce the amount of inerts in the system, which reduces the total purge necessary and minimizes operating costs. This will also change the selectivity to ethylene oxide from 65-75% for the air-based process to 85-90% for the oxygen-based process. This change alone will increase profits by $15 million per year. Another major process decision was to change the hydration process of ethylene oxide to ethylene glycol to include the use of a polystyrene based ion exchange resin as the catalyst. Although the use of an ion exchange resin as a catalyst is a new technology in this industry, it can increase the selectivity to monoethylene glycol over diethylene glycol from approximately 90% to 99%. Since monoethylene glycol sells for 38 c/lb while diethylene glycol sells for only 26c/lb. This change in selectivity will increase profits by $9.7 million per year, which is well worth the risk for utilizing a new technology. Overall, the capital investment for this project is estimated at $124 million. At an estimated production rate of 890 million pounds per year of ethylene glycol at full capacity, the plant will use 450 million pounds per year of ethylene provided by direct pipeline, and 380 million pounds per year of oxygen which will be generated by an oxygen generation plant. The anticipated Internal Rate of Return after the 16 year lifespan is expected to be 30%, with a break even period of 2 years. This process seams very feasible according to this preliminary economic analysis, but a few further studies can be done to improve the profitability further. This process can be optimized to reduce the overall amount of heating and cooling used in the process, which could potentially save a few million dollars per year, but would probably require a small increase in the capital investment. Also, the carbon dioxide stream exiting the CO2 stripper could be purified and sold to offset the cost of production for another few million dollars savings per year. One of the most important recommendations would be to lock in the price of ethylene by entering into a long term contract with the providers. A difference of 1 c/lb in the cost of ethylene would save almost $9 million per year. At this stage, our recommendation is to proceed with this project to the next stage of development. Table of Contents 1. Introduction ............................................................................................................................. 1 1.1. Background ..................................................................................................................... 1 1.2. Proposed Plant ................................................................................................................ 2 1.3. Ethylene Oxide Production ............................................................................................. 3 2. Design Basis............................................................................................................................ 7 2.1. Project Scope Summary .................................................................................................. 7 2.1.1. Products................................................................................................................... 7 2.1.2. Technology ............................................................................................................. 7 2.1.3. Location .................................................................................................................. 7 2.1.4. Plant Capacity ......................................................................................................... 7 2.1.5. Raw Materials ......................................................................................................... 7 2.1.6. Down Time ............................................................................................................. 7 2.2. Location of Plant ............................................................................................................. 8 2.3. Size of Plant .................................................................................................................... 9 2.4. Product Quality Specifications ..................................................................................... 10 2.5. Products Handled, Stored, and Shipped ........................................................................ 11 2.6. Raw Materials ............................................................................................................... 11 2.7. Major Process Descriptions & Assumptions ................................................................ 12 2.8. Ancillaries Design ......................................................................................................... 15 3. Process Description ............................................................................................................... 16 3.1. Oxygen Mixing Station ............................................................................................. 16 3.2. Ethylene Feed Prep ................................................................................................... 16 3.3. EO Reactor ................................................................................................................ 16 3.4. EO Absorber ............................................................................................................. 17 3.5. EO Stripper ............................................................................................................... 18 3.6. EG Reactor ................................................................................................................ 18 3.7. EG Dehydration ........................................................................................................ 19 3.8. EG Purification ......................................................................................................... 20 3.9. CO2 Absorber ............................................................................................................ 20 3.10. CO2 Stripper .......................................................................................................... 20 4. Process Flow Diagrams......................................................................................................... 21 5. Material & Energy Balance .................................................................................................. 33 6. Equipment ............................................................................................................................. 45 7. Plant Layout .......................................................................................................................... 61 8. Operating Requirements ....................................................................................................... 62 8.1. Utilities.......................................................................................................................... 62 8.2. Waste Streams ............................................................................................................... 64 9. Environmental and Safety Considerations ............................................................................ 65 9.1. Environmental Concerns ............................................................................................... 65 9.2. Safety Concerns ............................................................................................................ 66 9.3. Waste Minimization ...................................................................................................... 68 10. Economic Feasibility ........................................................................................................ 69 10.1. Economic Assumptions ............................................................................................ 69 10.2. Capital Equipment Costs........................................................................................... 70 10.3. Manufacturing Costs ................................................................................................. 72 10.4. Year-by-Year Economic Analysis ............................................................................ 76 10.5. Sensitivity & Cost Behavior Analysis ...................................................................... 78 11. Conclusions and Recommendations ................................................................................. 86 12. Appendices ........................................................................................................................ 71 12.5. Sample Calculations.................................................................................................. 72 12.6. MSDS........................................................................................................................ 73 12.7. Aspen Process Simulation......................................................................................... 74 13. Literature Cited ................................................................................................................. 75 EG Production 1 1. Introduction 1.1. Background Ehylene oxide is a highly versatile commodity chemical, which is used as an intermediate for the production of a variety of chemicals. It also kills bacteria, mold, and fungi, and is therefore used as a sterilant. It is a highly reactive colorless gas with a slightly sweet odor. Other names for it include E.O., oxirane, dimethylene oxide, and 1,2epoxyethane. During World War I, it gained importance in industry and was produced on a small scale for its use in the production of ethylene glycol, an engine coolant, and the chemical weapon mustard gas. Ethylene oxide was once used for the production of acrylonitrile but that was discontinued in 1966 (13). Ehylene glycol is a diol that is used as engine coolant, automotive antifreeze, and used to manufacture polyester PET (polyethylene terephthalate). Its higher boiling point allows for radiators to operate at higher temperatures. It can also be used as a chemical dehydrator for natural gas production. It is an odorless, colorless, syrupy liquid with a sweet taste. As little as a mouthful of antifreeze solution ingestion in either a child or adult may lead to toxic signs and symptoms. Other names for ethylene glycol include glycol; glycol alcohol; ethylene dehydrate, and 1,2-ethanediol (13). More than 65% of the ethylene oxide produced in the industry is used in the production of ethylene glycol and the remaining pure ethylene oxide is used as a sterilant for food, cosmetics, surgical equipment, and plastic devices that cannot be sterilized by steam. The major producers of ethylene oxide and ethylene glycol in North America are DOW Chemical (1,313,000 ton EG/year & 1,502,000 ton EO/year), Shell Chemicals (721,000 ton EG/year & 987,000 ton EO/year) and Huntsman (280,000 ton EG/year & 507,000 ton EG/year) (20). More than 65% of the ethylene oxide produced in the industry is used in the production of ethylene glycol and the remaining pure ethylene oxide is used as a sterilant Drexel University, CHE 483 Completed By: Reviewed By: EG Production 2 for food, cosmetics, surgical equipment, and plastic devices that cannot be sterilized by steam. The major producers of ethylene oxide and ethylene glycol in North America are DOW Chemical (1,313,000 ton EG/year & 1,502,000 ton EO/year), Shell Chemicals (721,000 ton EG/year & 987,000 ton EO/year) and Huntsman (280,000 ton EG/year & 507,000 ton EG/year) (20). The demand for ethylene glycol is increasing with the increase of the demand of its end use segments, like antifreeze additive, polyester fiber, PET bottles and film. As there has been no significant net addition of new capacity in the industry since 2001, there is a measurable shortfall in ethylene glycol supply. Overall, ethylene glycol is expected to increase by 6%-7% per year through 2010 (20). A small amount of diethylene glycol is made as a byproduct during the production of ethylene glycol as a byproduct. The diethylene glycol production usually exceeds demand; therefore, the price is generally low. It is used as an ingredient in unsaturated polyester resins and in polyurethane manufacturing. To minimize production of diethylene glycol, we have chosen to use a highly selective catalyst to convert the majority of ethylene oxide to monoethylene glycol. 1.2. Proposed Plant The proposed ethylene oxide plant is coupled with the production of ethylene glycol to maximize profits, minimize inherently dangerous inventories and increase safety in handling and transporting products. More than 98% of the ethylene oxide in the plant is converted to ethylene glycol and the remainder is converted to di-ethylene and tri-ethylene glycols. The plant is not producing pure ethylene oxide for sales and is not storing any ethylene oxide on-site because ethylene oxide is a very hazardous material to handle. The ethylene oxide vapor is very flammable and explosive; its flammability limit in air ranges from 3-100%. It requires pressurized storage tanks padded with nitrogen, low temperature, and to be kept away from all ignition sources or oxidizing agents. To eliminate the hazards, a dilute stream of ethylene oxide in water is fed to the hydrolysis Drexel University, CHE 483 Completed By: Reviewed By: EG Production 3 unit to make a safer product form ethylene glycol. In addition, the value of producing only ethylene glycol is higher than converting a portion of the production to ethylene glycol (18). 1.3. Ethylene Oxide Production Ethylene oxide was first prepared in 1859 by Charles Wutz using a potassium hydroxide solution to eliminate the hydrochloric acid from ethylene chlorohydrin. In 1914 the ethylene chlorohydrin process was the first technology to produce ethylene oxide commercially. The process involves the reaction of ethylene with hypochlorous acid followed by dehydrochlorination of the resulting chlorohydrin with lime to produce ethylene oxide and calcium chloride. Although the selectivity of this process was approximately 80%, the process itself was very inefficient and caused pollution problem by generating large quantity of unwanted chlorinated hydrocarbon byproducts (11). Chlorohydrin can be converted directly to ethylene glycol by hydrolysis with a base, generally caustic or caustic/ bicarbonate mix. This process is unattractive because it requires a difficult salt separation to purify the glycol (8). Union Carbide Corp. was the first to commercialize this process in the United States in 1925. In 1931, Theodore Lefort discovered a way to prepare ethylene oxide directly from ethylene and oxygen with a silver catalyst, the direct oxidation processes. This process was more economically competitive and soon replaced the ethylene chlorohydrin process (13). The direct oxidation technology is the dominant process to produce ethylene oxide commercially today. It utilizes the catalytic oxidation of ethylene with pure oxygen over a silver based catalyst to yield ethylene oxide. The direct oxidation technology is available from Dow Chemical, Nippon Shokubai, Scientific Design, and Shell. The proposed plant is using technology licensed by Shell, which minimizes the formation of unwanted products and increases the selectivity of the ethylene oxide. The main disadvantage of this process is that the per pass conversion is lower hence giving us more to recycle (reword this). An older version of the direct oxidation process uses air instead Drexel University, CHE 483 Completed By: Reviewed By: EG Production 4 of pure oxygen. Air is still used in some older ethylene oxide plants, but most air-based plants have been converted to use oxygen due the advantages. For all plant capacities, the oxygen-based reactor yields a higher selectivity and requires less catalyst (11). The average selectivity ranges from 65-75% for the air-based process compared to 85-90% for the oxygen-based process. Furthermore, the concentration of oxygen and ethylene in the reactor feed can be higher in oxygen-based plant, which improves the catalyst selectivity (11). Therefore, the overall yield of ethylene oxide for the oxygen-based process is more than an air-based process. The length of silver based catalyst life is an important parameter to consider due to its high cost. For the oxygen based oxidation process, the catalyst has a longer life and less catalyst is required per unit weight of feed. Typically the air-based oxidation requires 1.5 times the catalyst in oxygen oxidation process (12). The required amount of catalyst and length of catalyst life makes the oxygen-based process a more economically viable choice. The oxygen-based process may have a higher operating cost, but the initial capital costs are much lower compared to an air-based plant. The air-based process requires more catalyst, more reactors, air purification units, and a purge reactor system (12). This process introduces a large amount of inert gas into the recycle stream, which must be vented to maintain constant nitrogen concentration in the system. Consequently, the airbased process requires much higher initial construction cost for compression, piping, and waste gas handling system than the oxygen-based plant. Alternatively, the pure oxygenbased process reduces the quantities of inert gases introduced into the cycle. As a result, the majority of the unconverted ethylene is recovered from the system. Due to these reasons, the oxygen-based process is a more attractive choice. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 1.4. 5 Ethylene Glycol Ethylene glycol has been produced commercially by hydrolyzing ethylene carbonate, which is the product of reacting ethylene oxide with carbon dioxide. Each reaction requires different operating conditions causing a need for more than one reactor, which leads to higher capital and operating costs. Another method of producing ethylene glycol is by direct oxidation of ethylene to ethylene glycol which is done with acetic acid as the catalyst. The yield of ethylene glycol is greater than 90% which is higher than hydrolysis of ethylene carbonate. However, the acid catalyst has a higher separation and purification cost, and causes corrosion problems. Thus this method has been abandoned (9). Today the most common commercial source of producing ethylene glycol is by direct oxidation of ethylene to ethylene oxide followed by hydrolysis of the ethylene oxide to ethylene glycol. Widespread industrial production of ethylene glycol via this method began in 1937, when cheap ethylene oxide became available (13). The ethylene oxide is thermally hydrolyzed to ethylene glycol with either an acid or base catalysis or uncatalyzed in a neutral medium (9). If no catalyst is used with the reaction, a considerable amount of excess water must be used to inhibit the side reactions and prevent producing higher weight glycols. This increases the selectivity of ethylene glycol but a large amount of water must be removed in order to recover pure ethylene glycol. The excess water from the hydrolysis is removed in an evaporator and the ethylene glycol is refined by vacuum distillation. Such separation of large amounts of water from the product involves large expenditure and is economically unattractive (19). Many catalysts have been proposed and researched to optimize selectivity, lower the reaction temperature and reduced the amount of excess water required. The proposed ethylene glycol plant is using the technology licensed by Shell. (reference missing) This technology utilizes an anion ion-exchange catalyst, using a hydroxyl group as the anion, to maximize profits by providing higher selectivity, minimizing production of higher glycols, and reducing operating temperature and amount of excess water needed. This Drexel University, CHE 483 Completed By: Reviewed By: EG Production 6 process allows roughly 97% conversion of ethylene oxide to ethylene glycol. Also, the ion-exchange catalyst is relatively harmless and non-corrosive. The catalyst resin is solid and requires no separation equipment other than a catalyst bed screen. Due to these reasons, the anion ion-exchange catalyst based process is a more economically viable choice. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 7 2. Design Basis 2.1 Project Scope Summary 2.1.1. Products O Ethylene Glycol (MEG) O DiEthylene Glycol O TriEthylene Glycol 2.1.2. Technology O Shell Technology Catalyst for oxidizing ethylene O Ion Exchange Catalyst for Ethylene Glycol Reactor 2.1.3. Location O Port Arthur, Texas O Self sufficient unit including utilities adjacent to BASF/ATOFINA 2.1.4. Plant Capacity O 896 MM lb/yr 2.1.5. Raw Materials O Ethylene O Oxygen O Silver Based Catalyst (CRI catalysts) O Potassium Carbonate O Ion-exchange Catalyst O Sodium hydroxide 2.1.6. Down Time O One annual site-wide shutdown of up to 3 weeks for maintenance Drexel University, CHE 483 Completed By: Reviewed By: EG Production 2.2. 8 Location of Plant The proposed site of the ethylene oxide plant in conjunction with ethylene glycol plant is Port Arthur, Texas. It is adjacent to the BASF/ATOFINA steam cracker which produces 1.72 million lbs of ethylene per year (2). Ethylene will be directly transported by existing underground pipeline systems under high pressures from BASF/ATOFINA to the ethylene oxide process. Since the major expense for manufacture of ethylene oxide is the acquisition of ethylene, tapping into an existing pipeline and transferring ethylene approximately 5 miles from BASFT/ATOFINA will significantly reduce the cost of transportation and expedite delivery time. See Figure 1-1 and Figure 1-2 for plant location. Also, the contract between BASF/ATOFINA and GLYDE Company will include a guaranteed continual supply of ethylene to eliminate the need for ethylene storage and we plan to synchronize the shut down schedule of our ethylene oxide plant with the steam cracker to avoid any unnecessary downtime. Figure 1-1: Port Arthur, TX Figure 1-2: GLYDE & BASF location in Port Arthur, TX The proposed plant will be a grass-roots site, and will be a self sufficient unit including all utilities and a waste water treatment plant. Since the average annual production is 880 million lbs per year, the most economical transportation route can be accomplished by either ground, marine, or railway transportation. Port Arthur is located approximately 15 miles southeast of Beaumont and Interstate-10, a major road that Drexel University, CHE 483 Completed By: Reviewed By: EG Production 9 connects other large transportation routes such as I-110 and I-95. Also, it is located 95 miles east of Houston, 280 miles from San Antonio, 25 miles from the Louisiana border and 260 miles from New Orleans. Port Arthur is near major ports and major highways entrances, which allows us to cheaply transport both raw materials and final products. In addition, Port Arthur offers proximity and growth opportunity in the ethylene glycol market. Port Arthur’s winter temperatures average in the mid 60s, while summers are warm with monthly averages in the low 80s. The highest temperature is 105°F and the lowest is 30°F(check). The typical wind speed in Port Arthur arranges between 7.1 knots and 11.6 knots in the direction of south or southeast. These warm temperatures reduce the need to insulate pipes or reactors to prevent massive heat loss. However, we still need to consider some insulation for the heat loss due to wind. We also need to consider the relative humidity to determine the highest temperature for cooling water, where the average relative humidity ranges from 75 % to 80.5%. 2.3. Size of Plant The proposed plant is designed to produce approximately 880 million lbs per year of ethylene glycol at 99.8% purity and 2.2 million lbs per year of diethylene glycol at 99.6% purity based on 8100 hours of operation per year. Approximately 484 million lbs per year of ethylene at 98% purity and 370 million lbs per year of oxygen at 99% purity are required to produce the expected quantity of products. The design operating rate is 108,000 lbs per hour, which is 10% higher than the hourly average annual rate to compensate any unexpected downtime or maintenance. The anticipated downtimes include one annual site-wide shutdown of up to 3 weeks for maintenance and an extra shutdown every 3 years for ethylene oxide and ethylene glycol catalyst recharging. Today approximately 15.6 million tons of ethylene glycol is produced worldwide per year and 20% of this market is produced in United States. Worldwide Ethylene OxideEthylene Glycol (EO-EG) market has tightened significantly. World wide demand growth has outpaced capacity increments. Prices are the highest level in 15 years. World Drexel University, CHE 483 Completed By: Reviewed By: EG Production 10 wide the demand for EG is expected to increase by 6.5%-7% or approximately 1 million m.t., far exceeding capacity additions. EG demand is expected to increase by 6%-7% through 2110.(20) GLYDE will be capturing 13% of the US market and 2.5% of the world market. 2.4. Product Quality Specifications More than 50% of the total ethylene glycol produced goes into manufacturing polyester, mostly PET (polyethylene terephthalate), and the majority of the rest is used in antifreeze solutions for automotive applications (20). PET applications include fibers, resins, and films. PET grade monoethylene glycol requires at least 99.8% purity, no more than 0.10 wt% of diethylene glycol and less than 0.10 wt% of water content. The industrial grade ethylene glycol is more suitable for manufacturing of antifreeze, which requires a lower purity of 98.0%, no more than 0.50 wt% of diethylene glycol and less than 0.50 wt% of water. Diethylene glycol requires approximately 99.6% of purity and less than 0.20 wt% of water. See Table 2-3 for the summary of product quality specifications. Table 2-3: Product Quality Specifications(12) MonoEthylene Glycol Property Polyester Fiber Grade Antifreeze Grade Diethylene Glycol Purity, % >99.8 >98.00 Diethylene Glycol Content, wt % <0.10 <0.50 Boiling Range (101.3 kPa), °C 196-199 195-200 242-247 Density (20 °C), g/cm 1.1135-1.1140 1.113-1.115 1.1160-1.1175 Refractive Index 1.4315-1.4320 1.431-1.433 1.4460-1.4475 Water Content, wt% <0.100 <0.50 <0.20 3 Drexel University, CHE 483 Completed By: 99.6 Reviewed By: EG Production 2.5. 11 Products Handled, Stored, and Shipped Ethylene glycol and diethylene glycol are transported in railway tank car, having a total capacity of 10600 gl (chk this). The interior surfaces are finished with a protective material that will not deteriorate the products’ quality. Also, the consumer can supply its own road tank cars for transportation. Ethylene glycol and diethylene glycol are stored in enclosed storage tanks made of acid-resistant or stainless steels and under a protective nitrogen blanketing. When the temperature is less that 25-30 °C, it is permissible to store both products without nitrogen blanketing. In addition, these must not be stored with oxidizing agent. 2.6. Raw Materials The following raw materials are required for the production of ethylene oxide, ethylene glycol and diethylene glycol. o Ethylene: 98% Purity (2% ethane & methane) Receive in gas form by existing high-pressure (370 psia) underground pipeline No on-site storage o Oxygen: 99% Purity (1% nitrogen, argon) Receive in gas form by pipeline from a satellite plant owned/operated by air plant vendor such as Praxair No on-site storage o Silver-Based Catalyst: Supplier: CRI Cannot be regenerated Ship spent catalyst to outside contractor to recover silver Receive in bulk via road tanker No on-site storage o DOWTherm (Heat Transfer Oil) Drexel University, CHE 483 Completed By: Reviewed By: EG Production 12 Supplier: DOW Chemical in Houston, TX Receive in bulk via road tanker Store in a dedicated isolated storage building o Potassium Carbonate (CO2 scrubber): Concentration: 15 mol% in water Supplier: Cost Chemicals, Inc. (Coast Southwest) in Dallas, TX Receive in bulk via road tanker Store in a storage building o Anion Ion-exchange Catalyst (OH- form) Supplier: Sybron Chemicals Inc. Start up requires washing the resin with CO2 saturated water and CO2 gas and washing with water. Can be regenerated on-site every 3 years or so Receive in bulk via road tanker No on-site storage o NaOH Supplier: The Boyer Corporation Shipping form: solid either beads or flakes Depending on the amount needed shipped in bulk via road tanker. 2.7. Major Process Descriptions & Assumptions The following assumptions and process decisions are made based on literature data and current industry practices. o Overall heat transfer coefficient of 150 btu/hr sqft oF for liquid to liquid transfer or in the presence of a boiling or condensing fluid, and 75 btu/hr sqft oF elsewhere. o Pressure drop of 6 psi for positive pressure heat exchangers, 3 psi or less for vacuum heat exchangers, and 15 psi for packed bed reactors. o Cooling Water Temperature comes into the process at 86 oF and leaves at 120 oF. o The efficiency of the CO2 absorber is set to 85% CO2 removal by adjusting the liquid flow rate. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 13 o The oxygen that is used must be extremely pure (>99%) to maintain high selectivity in ethylene oxide reactor. Oxygen is added in a special mixing device that ensures rapid homogenization with the recycle gas. This is necessary because the explosive limit is locally exceeded at the mixing point (12). o The ethylene conversion is approximately 12.5%, and its catalyst selectivity’s are 90% ethylene oxide, 9.9% carbon dioxide and 0.1% others. o The typical lifetime of the silver-based catalyst is two to five years, depending on the type of catalyst, the rate of ethylene oxide production, and the purity of the reaction gases (11). Since the production rate of ethylene oxide is approximately 880 million lbs per year using high-selectivity catalysts and pure oxygen, we expect the lifetime of the catalyst to be approximately 3 years. o A large amount of heat is released by the oxidation of ethylene since this reaction is highly exothermic. Consequently, the energy recovery and its integration are prime concerns in a process design. We decided to use an oil-cooled reactor, which removes the heat of reaction by circulating hot oil such as DOWTherm on the shell side. Then, the hot oil is cooled in a steam generator, producing considerable amounts of high pressure steam for the ethylene glycol production and other processes at the plant site. DOWTherm has properties at different temperature ranges as shown on Table 2-6. Table 2-6: DOWTHERM Properties(23) Temperature Specific Heat Density Thermal Conductivity 3 Btu/hr ft 2 Viscosity Vapor Pressure cP Psia ºF Btu/ lb ºF lb/ft 50 0.380 64.76 0.0766 88.17 150 0.420 62.33 0.0725 6.10 250 0.459 59.88 0.0683 1.94 350 0.499 57.38 0.0642 0.99 0.09 450 0.538 54.82 0.060 0.62 0.71 550 0.578 52.18 0.0558 0.43 3.49 650 0.617 49.41 0.0517 0.32 12.20 o We decided to use a packed-bed multi-tubular reactor instead of a fluidized bed reactor or quench bed reactor even though oxidation of ethylene is highly exothermic. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 14 Also, no such reactors are used currently on a commercial basis since there is no benefit in maximizing the selectivity and led to problems such as abrasion and sintering (11). The standard practice in the industry is to employ packed-bed multitubular reactors. o We decided to build one ethylene oxide reactor instead of two small reactors in parallel. This will significantly reduce the construction and maintenance costs. Also, we plan to leave enough space in the plant for a possible expansion to the site in the future. o The temperature or the pressure of ethylene oxide reactor must not exceed 570 ºF or 290 psia in order to sustain catalyst activity (11). o At the present time, there is no known method to regenerate silver-based catalyst. Therefore, we will be shipping spent catalysts to an outside source to recover silver. o The ethylene oxide conversion is 98% and its catalyst selectivities are 98% monoethylene glycol and 2% diethylene and tri ethylene glycol. o The ethylene glycol reactor will be a packed bed gas phase reactor with a residence time of approximately 20-30 seconds. The residence time is an assumption based on lab data for the ion-exchange catalyst (19). o At this time the ion-exchange catalyst has not been used on a large scale, however lab tests have shown that it doesn’t significantly deteriorate over time. The catalyst will need to be regenerated every three to four years. The regeneration can be done simply and cheaply by either current or co-current washing with sodium hydroxide (NaOH) and then rewashing with carbon dioxide (CO2), or washing with saturated water and then rewashing with CO2 gas. o The ethylene glycol catalyst restricts the reactor temperature to not exceed 320 oF with a maximum pressure loss of 3 bar. o By-Products Carbon dioxide: The CO2 rich waste generated from the ethylene oxide reactor (R-101) will be sent to a flare. Purge stream from EO stripper (T-201): The purge stream containing mostly ethylene, CO2, and impurities such as argon and ethane/methane will be sent to a flare. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 2.8. o 15 Ancillaries Design The plant plot is a rectangular shape and has easy access to roads, railroads, and water. The prevailing winds blow in the south, south-east direction. o The control room and lab are located at the west side of the plant. This will form the process area. The main offices and parking lot are adjacent to the lab and give employees easy access to the main roads. o The oxygen plant, oxygen mixing station and the ethylene prep station are located at the south-east end of the plant. The location of these components takes into account the wind direction and is far away from the control room to minimize human casualty in the event of an explosion. o The loading station is located on the southern end of the plant and enables easy loading and unloading of materials due to its proximity to the railroad tracks. o The maintenance building and warehouse are located in the north-western end of the plant above the main office area. o Our production equipment is located in the main process plant which starts from the northeastern boundary and extends into the south eastern side ending before the oxygen plant area. All our equipment is laid out on concrete slabs. o The main flare for our plant is located on the south-western end of the facility across the railroad tracks. The flare has a circular boundary around it at a radius of 75 ft., which is considered no-mans land. o The wastewater plant and utilities building are located adjacent to the flare on the south eastern tip of the facility. o The railroad tracks located in the south end of the facility will be laid out by us and will connect to major railroad systems. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 3. 16 Process Description 3.1. Oxygen Mixing Station An outside contractor such as Praxair will be responsible for running the oxygen generating plant on site to separate the oxygen from air and allowing high purity oxygen to be produced. The oxygen that is used must be extremely pure (>99%) and is obtained by air separation. This pure oxygen is added in a special mixing device that ensures rapid homogenization with the compressed recycle gas from the EO stripper (T-201) and carbon dioxide absorber (T-301) details of which are proprietary. This is necessary because the explosive limit is locally exceeded at the mixing point (11). 3.2. Ethylene Feed Prep Ethylene is continuously fed to our process through underground piping from BASF/ATOFINA at 86 °F and 353 psig. To achieve operating condition, ethylene is processed through a turbine to recover power (175 KW) while reducing the pressure to 220 psig.Then, the mixture of ethylene recycle gas and oxygen is fed through a pre-heat exchanger (E-101) to reach the temperature of 302 oF. 3.3. EO Reactor Ethylene and oxygen are fed in roughly stoichiometric proportions in the gas phase to the EO reactor (R-101), where ethylene is oxidized with oxygen over a silverbased catalyst to yield ethylene oxide. This packed-bed multi-tubular reactor consists of large bundles of several thousand tubes that are 20ft long and have an internal diameter of 1 inch. The silver-based catalyst is packed in the tubes in the form of spheres with a diameter of 0.27 inches (11). This system allows ethylene oxide selectivity of approximately 90% and the ethylene conversion of 12.5% (11). The reaction has a residence time of 0.2s.. In addition, the EO reactor is integrated with an oil system that circulates through both the reactor and reactor trim cooler (E-102) to remove the heat of reaction since the oxidation of ethylene is highly exothermic. Then, the hot oil is used to Drexel University, CHE 483 Completed By: Reviewed By: EG Production 17 provide heat elsewhere in process. Also, the pressure of the EO reactor is controlled by the flow rate of the purge stream after the EO absorber (T-201). Ethylene is reacted at 230 °F and 118 psig to produce ethylene oxide, carbon dioxide, water, and heat as well as small amounts of acetaldehyde and formaldehyde. The main reaction, formation of ethylene oxide from ethylene, is as follow: O H2C CH2 1/2 O O H2C CH2 Molecular oxygen is adsorbed to the silver surface of catalyst and reacts with ethylene to H2C CH2 O 3 O 2H 2 O C O H form ethylene oxide. With only 12.5% of ethylene conversion per pass, majority of O unreacted O ethylene and oxygen will be recycled back into the feed. H2C 2½ O CH2 2 O O C O O 2H O H The carbon H2Cbyproducts, CH2 O and water, are either formed by complete combustion of 1/2 Odioxide H2C O CH2 ethylene: HO H2C O CH2 O 1/2 O HC CHH2 HH 2C 2C CH CH 22 3 O H 2 H2C O CH2 O 3 O or by further oxidation of ethylene oxide: O 2½ O O H2C O CH2 HO H2 C C H2C H2 CH2 O OH To prevent further H2C O CH2 2½ O CH3 CH2 H 2 O C O C O 2H 2H C O O2 H 2 O OH 2 H HO O O O O OH H H H OH H HO H2 C C is added to the inlet of oxide, ethylene dichloride H2 OH HO H2 OH of ethylene to carbon dioxide and improve selectivity. C C H H2 OH OH H of ethylene O the reactor to inhibit the oxidation H2C 2 C O 2 O O H H oxidation 2 O C H C2 H OO O O HO OH H2 3.4.C EO O C Absorber H2 O HO HO OH H2 OH CH H C O C oxide is recovered in the3 EO absorber (T-201) by scrubbing Ethylene with water H2 OH at 176 oF. The absorber (T-201) theoretical stages. Ethylene oxide produced in the H has 12CH 3 HO reactor (R-101) and unreacted ethylene oxide recovered from EG dehydrator (T-501) overhead goes through the shell side of EO absorber liquid cooler (E-201) to achieve the operating temperature. Approximately 2800 gpm of makeup water mixed with the Drexel University, CHE 483 Completed By: Reviewed By: EG Production 18 recovered process water from the bottom of the EO stripper (T-202) enter the absorber from the top. Ethylene oxide is absorbed into the water along with some nitrogen and carbon dioxide, and traces of ethylene, ethane, and aldehydes. The aqueous stream is removed from the base of the absorber and sent to EO stripper (T-202). A small amount of the vapor product from the absorber is purged to a flare to remove impurities and prevent the buildup of inert compounds. Part of the stream is sent to carbon dioxide absorber (T-301), and the remainder is sent directly back to the reactor system as a recycle stream. 3.5. EO Stripper The liquid product containing mostly water and ethylene oxide from EO absorber (T-201) is heated to operating temperature of 325 oF in EO stripper pre-heater (E-204) then sent to the flash drum (V-201) where the lights are flashed off and the remaining liquid is sent to the EO stripper (T-202). The stripper (T-202) has 12 theoretical stages. In the stripper partially purified ethylene oxide is separated overhead and mixed with the vapor from the flash drum (V-201) after which it is sent to EG reactor for production of ethylene glycol. As mentioned earlier, the water recovered as a bottom product and cooled through a series of heat exchangers before it is recycled back to the EO absorber. 3.6. EG Reactor The mixture of ethylene oxide and water from the EO stripper (T-202) has a ratio of water to ethylene oxide of 4:1. This is fed into the EG reactor (R-401) at 118 psig and 194 oF. This packed-bed reactor contains the anion ion-exchange catalyst which are spherical shaped polystyrene gels with bicarbonate as the anion. It is packed in the reactor in the form of spheres with a diameter of .65 mm (19). The reactor has a residence time of 4 seconds and an overall conversion of ethylene oxide to glycols of 98%, with 98% selectivity of monoethylene glycol while the other 2% is di-ethylene glycol. Drexel University, CHE 483 Completed By: Reviewed By: H2C CH2 EG Production H2C CH2 The O 1/2 O 3 O O H2C CH2 19 O 2 O C 2H O H O reaction that takes place between the ethylene oxide and water is in the vapor 2 H glycol from C O of ethylene H phase and is strongly exothermic. 2 O formation OThe main reaction, 2½ O H2C O CH2 ethylene oxide, is as follow: O H2C HO H CH2 O C H2 H H2 C OH OH This formation is a nucleophilic substitution reaction involving the opening of the O HO HO (19). Because monoethylene OH ethylene oxide H2 ring by a nucleophile, in this case water C O C glycol H also acts as a nucleophile, it reacts with the ethylene oxide in the same way as 2 OH CH3below: H as shown water to form diethylene glycol To prevent a large formation of di- and triethylene glycols the anion in the catalyst and ethylene oxide react to form an adduct. The formation of the adduct competes with the reaction between monoethylene glycol and ethylene oxide reducing the formation of higher glycols. It is later hydrolyzed into ethylene glycol. The selectivity to monoethylene glycol is increased without having to supply excess water. 3.7. EG Dehydration The mixture of water and ethylene glycol leaving the glycol reactor (R-401) is sent through a flash drum (V-401) to remove the lights after which it is sent to the EG dehydrator (T-501) for purification. The dehydrator operates at 192oF and atmospheric pressure. The liquid removed from the top of the dehydrator partial condenser (E-501) is recycled back into the EO absorber (T-201) to provide absorbing water. The vapor from the partial condenser is sent through a compressor (C-501) to recover any unreacted ethylene and mixed with the EO reactor (R-101) effluent fed to the EO absorber. The H2O concentration at the bottom is 410 ppm.The bottom stream from the EG dehydrator is sent on through a series of distillation columns where the glycols are further separated from each other. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 3.8. 20 EG Purification The column operates under vacuum at a pressure of 1.5psia or less to lower the boiling point at the base of the column. The MEG purification column (T-502) is designed to remove monoethylene glycol overhead. Diethylene glycol is removed in the side stream. A mixture of Diethylene glycol and Triethylene glycol is removed from the bottoms. This column has 20 theoretical stages. The monoethylene glycol removed from the top of the column and the Diethylene glycol removed from the side is 99.9% pure ethylene glycol and is pumped down stream to the storage tanks. The bottoms stream containing mainly diethylene glycol and triethylene glycol is mixed with the distillate to eliminate off-spec material that could not be otherwise sold while maintaining the purity of the final MEG product. 3.9. CO2 Absorber The effluent gas from the EO absorber (T-201) mostly consists of CO2, ethylene, and impurities such as ethane and methane. A portion of the gas is sent to the CO2 absorber column (T-301), operating at 176 psig. This column has 12 theoretical stages. The carbonate solution consist of 11 wt.% potassium carbonate and 13 wt.% potassium bicarbonate is pumped to the top of the column to absorb CO2 from the gas and leaves at the bottom of the absorber. The vapor product from the top of the CO2 absorber is mixed back into the recycle stream which is fed into the EO reactor. 3.10. CO2 Stripper The CO2 rich carbonate solution is sent through the CO2 stripper economizer (E301) where it is heated upto 214 oF and then pumped into the top of CO2 stripper (T-302) column, where the solution is heated to 221 °F at 2 psig in order to separate the carbonate solution from the CO2. The CO2 Stripper column has 8 theoretical stages. The solution is collected at the bottom is cooled down in the stripper economizer and recycled back into the CO2 absorber (T-301) while the CO2 rich gas leaves the top of the stripper column to a flare. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 4. 21 Process Flow Diagrams LEGEND Plant Lettering Plant Areas Drawing No’s P-000 Pump 100 EO Reaction Section 100-A Feed Prep T-000 Column 200 EO Removal Section 100-B EO Reaction 300 CO2 Removal Section 200-A EO Absorber 400 EG Reaction Section 500 EG Purification Section V-000 Vessel C-000 Compressor E-000 Heat Exchanger X-000 Special Ancillaries Main Process Flow EO Stripper 300-A CO2 Absorber 300-B CO2 Stripper 400 EG Reaction 500-A EG Dehydrator 500-B MEG Purification 500-C MEG Recovery 500-D DEG Purification 600 Oil System Temperature (°F) Process Flow Control Line 200-B Pressure (psig) TIC 500 Temperature Indicating Controller Utility PC 500 Pressure Controller Stream Number PI 500 Pressure Indicator Flow Rate (lb/hr) FC Flow Controller 500 Drexel University, CHE 483 Completed By: Reviewed By: EG Production 22 Insert PFD’s Drexel University, CHE 483 Completed By: Reviewed By: EG Production 5. 33 Material & Energy Balance MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description 101 102 103 Compiled by: NPM Checked by: CHM 106 104 105 Ethylene Recycle From Ethylene Feed to E-101 602,615 191 221 590,077 0 590,077 1.00 lb/hr wt% 133 0.0002 21,541 0.0357 77,142 0.1280 0 0.0000 0 0.0000 73 0.0001 841 0.0014 442,848 0.7349 31 0.0001 8,032 0.0133 293 0.0005 248 0.0004 51,436 0.0854 0 0.0000 Ethylene Pipeline Feed Ethylene Process Feed Air Feed to O2 Plant O2 Process Feed Ethylene Recycle From OMS to Ethylene Feed 55,309 86 353 30,928 0 30,928 1.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 11 0.0002 55,293 0.9997 0 0.0000 0 0.0000 4 0.0001 0 0.0000 0 0.0000 0 0.0000 55,309 53 221 45,419 0 45,419 1.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 11 0.0002 55,293 0.9997 0 0.0000 0 0.0000 4 0.0001 0 0.0000 0 0.0000 0 0.0000 195,134 86 0 2,651,198 0 2,651,198 1.00 lb/hr wt% 0 0.0000 350 0.0018 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 149,417 0.7657 45,368 0.2325 0 0.0000 45,722 86 221 34,969 0 34,969 1.00 lb/hr wt% 0 0.0000 350 0.0076 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 4 0.0001 45,368 0.9923 0 0.0000 547,307 206 221 544,560 0 544,560 1.00 lb/hr wt% 133 0.0002 21,541 0.0394 77,142 0.1409 0 0.0000 0 0.0000 73 0.0001 829 0.0015 387,553 0.7081 31 0.0001 8,032 0.0147 289 0.0005 248 0.0005 51,436 0.0940 0 0.0000 Stream Properties Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows ACETALD ARGON CARBON DIOXIDE DEG EG EO ETHANE ETHYLENE FORM WATER METHANE NITROGEN OXYGEN TEG Drexel University, CHE 483 Completed By: Reviewed By: EG Production 34 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description 107 EO Reactor Pre-Heater to EO Reactor 108 109 EO Reacgtor to EO Trim EO Trim Cooler to EO PreCooler Heater Compiled by: NPM Checked by: CHM 201 110 111 EO Trim Cooler to EO Absorption Section Ethylene Recycle from Recycle Compressor EO Rich Ethylene and Lights from C-501 to EO Asorber Gas Cooler 601,490 289 184 768,233 0 768,233 1.00 lb/hr wt% 222 0.0004 20,394 0.0339 92,643 0.1540 0 0.0000 0 0.0000 78,314 0.1302 771 0.0013 388,092 0.6452 44 0.0001 14,343 0.0238 262 0.0004 238 0.0004 6,167 0.0103 0 0.0000 501,586 212 221 506,666 0 506,666 1.00 lb/hr wt% 133 0.0003 21,191 0.0422 77,142 0.1538 0 0.0000 0 0.0000 73 0.0001 829 0.0017 387,553 0.7727 31 0.0001 8,032 0.0160 289 0.0006 244 0.0005 6,068 0.0121 0 0.0000 633,146 294 184 821,099 0 821,099 1.00 lb/hr wt% 1,245 0.0020 20,867 0.0330 101,086 0.1597 0 0.0000 630 0.0010 80,845 0.1277 781 0.0012 394,201 0.6226 277 0.0004 26,245 0.0415 264 0.0004 245 0.0004 6,461 0.0102 0 0.0000 Stream Properties 602,615 302 213 713,106 0 713,106 1.00 lb/hr wt% 133 0.0002 ACETALD 21,541 0.0357 ARGON 0.1280 CARBON DIOXIDE 77,142 0 0.0000 DEG 0 0.0000 EG 73 0.0001 EO 841 0.0014 ETHANE 442,848 0.7349 ETHYLENE 31 0.0001 FORM 8,032 0.0133 WATER 293 0.0005 METHANE 248 0.0004 NITROGEN 51,436 0.0854 OXYGEN 0 0.0000 TEG Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows Drexel University, CHE 483 601,490 482 198 899,263 0 899,552 1.00 lb/hr wt% 222 0.0004 20,394 0.0339 92,643 0.1540 0 0.0000 0 0.0000 78,314 0.1302 771 0.0013 388,092 0.6452 44 0.0001 14,343 0.0238 262 0.0004 238 0.0004 6,167 0.0103 0 0.0000 601,490 392 191 842,634 0 842,634 1.00 lb/hr wt% 222 0.0004 20,394 0.0339 92,643 0.1540 0 0.0000 0 0.0000 78,314 0.1302 771 0.0013 388,092 0.6452 44 0.0001 14,343 0.0238 262 0.0004 238 0.0004 6,167 0.0103 0 0.0000 Completed By: Reviewed By: EG Production 35 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description Compiled by: NPM Checked by: CHM 207 202 203 204 205 206 EO Absorber Gas Cooler to EO Absorber Liquid from EO Absorber to Liquid Cooler Absorber Liquid Cooler to Absorber Gas Cooler Absorber Gas Cooler to Stripper Pre-Heater Pre-Heater to Flash Vessel Liquid from Flash to Stripper Feed Pump Stream Properties 633,146 253 176 806,493 0 806,493 1.00 lb/hr wt% 1,245 0.0020 ACETALD 20,867 0.0330 ARGON 0.1597 CARBON DIOXIDE 101,086 0 0.0000 DEG 630 0.0010 EG 80,845 0.1277 EO 781 0.0012 ETHANE 394,201 0.6226 ETHYLENE 277 0.0004 FORM 26,245 0.0415 WATER 264 0.0004 METHANE 245 0.0004 NITROGEN 6,461 0.0102 OXYGEN 0 0.0000 TEG Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows Drexel University, CHE 483 1,224,356 220 176 32,520 158,007 11,398 0.01 lb/hr wt% 1,352 0.0011 480 0.0004 8,425 0.0069 4 0.0000 23,345 0.0191 81,324 0.0664 10 0.0000 6,110 0.0050 298 0.0002 1,102,719 0.9007 2 0.0000 7 0.0000 281 0.0002 0 0.0000 1,224,356 248 169 41,187 160,122 19,781 0.01 lb/hr wt% 1,352 0.0011 480 0.0004 8,425 0.0069 4 0.0000 23,345 0.0191 81,324 0.0664 10 0.0000 6,110 0.0050 298 0.0002 1,102,719 0.9007 2 0.0000 7 0.0000 281 0.0002 0 0.0000 1,224,356 255 162 45,311 160,584 23,844 0.01 lb/hr wt% 1,352 0.0011 480 0.0004 8,425 0.0069 4 0.0000 23,345 0.0191 81,324 0.0664 10 0.0000 6,110 0.0050 298 0.0002 1,102,719 0.9007 2 0.0000 7 0.0000 281 0.0002 0 0.0000 Completed By: 1,224,356 325 162 127,647 162,339 105,946 0.04 lb/hr wt% 1,352 0.0011 480 0.0004 8,425 0.0069 4 0.0000 23,345 0.0191 81,324 0.0664 10 0.0000 6,110 0.0050 298 0.0002 1,102,719 0.9007 2 0.0000 7 0.0000 281 0.0002 0 0.0000 1,141,006 318 132 21,212 158,673 0 0.00 lb/hr wt% 909 0.0008 28 0.0000 67 0.0001 5 0.0000 23,278 0.0204 44,692 0.0392 0 0.0000 223 0.0002 217 0.0002 1,071,561 0.9391 0 0.0000 1 0.0000 25 0.0000 0 0.0000 Reviewed By: EG Production 36 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description 208 211 212 Feed Pump to EO Stripper EO Stripper Distillate Vapr From EO Flash 147,888 344 132 387,170 0 387,170 1.00 lb/hr wt% 909 0.0061 28 0.0002 67 0.0005 0 0.0000 0 0.0000 44,692 0.3022 0 0.0000 223 0.0015 217 0.0015 101,726 0.6879 0 0.0000 1 0.0000 25 0.0002 0 0.0000 83,675 318 132 168,271 0 168,271 1.00 lb/hr wt% 535 0.0064 426 0.0051 8,368 0.1000 0 0.0000 30 0.0004 36,649 0.4380 9 0.0001 5,888 0.0704 102 0.0012 31,399 0.3753 2 0.0000 6 0.0001 262 0.0031 0 0.0000 215 216 Compiled by: NPM Checked by: TAS 217 Bottoms from EO Stripper Recycle Pump to Stripper Pre-Heater to Liquid Cooler to Recycle Pump Pre-Heater Stream Properties 1,141,006 318 133 21,212 158,673 0 0.00 lb/hr wt% 909 0.0008 ACETALD 28 0.0000 ARGON 67 0.0001 CARBON DIOXIDE 5 0.0000 DEG 23,278 0.0204 EG 44,692 0.0392 EO 0 0.0000 ETHANE 223 0.0002 ETHYLENE 217 0.0002 FORM 1,071,561 0.9391 WATER 0 0.0000 METHANE 1 0.0000 NITROGEN 25 0.0000 OXYGEN 0 0.0000 TEG Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows Drexel University, CHE 483 992,984 358 134 18,913 141,479 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 4 0.0000 23,093 0.0233 0 0.0000 0 0.0000 0 0.0000 0 0.0000 969,887 0.9767 0 0.0000 0 0.0000 0 0.0000 0 0.0000 Completed By: 992,984 359 201 18,917 141,505 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 4 0.0000 23,093 0.0233 0 0.0000 0 0.0000 0 0.0000 0 0.0000 969,887 0.9767 0 0.0000 0 0.0000 0 0.0000 0 0.0000 992,984 260 201 17,566 131,404 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 4 0.0000 23,093 0.0233 0 0.0000 0 0.0000 0 0.0000 0 0.0000 969,887 0.9767 0 0.0000 0 0.0000 0 0.0000 0 0.0000 Reviewed By: EG Production 37 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description Compiled by: NPM Checked by: TAS 301 218 219 220 221 222 Liquid Cooler to Absorber Trim Cooler Trim Cooler to Water Recycle Liquid Feed to EO Absorber Absorber Make-up Water Vapor Product from EO Absorber Purge Stream from EO Absorber 23,310 86 172 372 2,781 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 23,310 1.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 515,742 248 172 682,863 0 682,863 1.00 lb/hr wt% 167 0.0003 21,219 0.0411 92,842 0.1800 0 0.0000 0 0.0000 81 0.0002 830 0.0016 388,087 0.7525 31 0.0001 5,876 0.0114 289 0.0006 244 0.0005 6,076 0.0118 0 0.0000 686 248 172 908 0 908 1.00 Stream Properties 992,984 226 194 17,164 128,397 0 0.00 lb/hr wt% 0 0.0000 ACETALD 0 0.0000 ARGON 0 0.0000 CARBON DIOXIDE 4 0.0000 DEG 23,093 0.0233 EG 0 0.0000 EO 0 0.0000 ETHANE 0 0.0000 ETHYLENE 0 0.0000 FORM 969,887 0.9767 WATER 0 0.0000 METHANE 0 0.0000 NITROGEN 0 0.0000 OXYGEN 0 0.0000 TEG Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows Drexel University, CHE 483 992,616 176 172 16,627 124,381 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 4 0.0000 22,721 0.0229 0 0.0000 0 0.0000 0 0.0000 0 0.0000 969,890 0.9771 0 0.0000 0 0.0000 0 0.0000 0 0.0000 1,106,173 175 172 18,533 138,635 0 0.00 lb/hr wt% 379 0.0003 0 0.0000 5 0.0000 4 0.0000 22,721 0.0205 562 0.0005 0 0.0000 3 0.0000 84 0.0001 1,082,412 0.9785 0 0.0000 0 0.0000 1 0.0000 0 0.0000 Completed By: lb/hr 0 28 123 0 0 0 1 516 0 8 0 0 8 0 Reviewed By: wt% 0.0003 0.0411 0.1800 0.0000 0.0000 0.0002 0.0016 0.7525 0.0001 0.0114 0.0006 0.0005 0.0118 0.0000 EG Production 38 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description 302 303 Ethylene Recycle 304 Ethylene Recycle Feed to CO2 Absorber Vapor Feed Recycle Compressor Compiled by: NPM Checked by: TAS 307 305 306 CO2 Absorber Vapor Product CO2 Absorber Liquid Product Liquid to CO2 Strippe Feed Pump 89,541 239 148 143,421 0 143,421 1.00 304,697 194 156 15,075 28,246 11,299 0.02 304,697 236 148 21,222 28,605 17,398 0.03 Stream Properties Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows 515,056 248 172 681,955 0 681,955 1.00 lb/hr 167 ACETALD 21,191 ARGON CARBON DIOXIDE 92,718 0 DEG 0 EG 81 EO 829 ETHANE 387,570 ETHYLENE 31 FORM 5,868 WATER 289 METHANE 244 NITROGEN 6,068 OXYGEN 0 TEG 0 K2CO3 0 KHCO3 Drexel University, CHE 483 wt% 0.0003 0.0411 0.1800 0.0000 0.0000 0.0002 0.0016 0.7525 0.0001 0.0114 0.0006 0.0005 0.0118 0.0000 0.0000 0.0000 412,044 248 172 545,564 0 545,564 1.00 lb/hr 133 16,953 74,175 0 0 65 664 310,055 25 4,694 231 195 4,855 0 wt% 0.0003 0.0411 0.1800 0.0000 0.0000 0.0002 0.0016 0.7525 0.0001 0.0114 0.0006 0.0005 0.0118 0.0000 0.0000 0.0000 103,011 248 172 136,391 0 136,391 1.00 lb/hr 33 4,238 18,544 0 0 16 166 77,514 6 1,174 58 49 1,214 0 wt% 0.0003 0.0411 0.1800 0.0000 0.0000 0.0002 0.0016 0.7525 0.0001 0.0114 0.0006 0.0005 0.0118 0.0000 0.0000 0.0000 lb/hr 0 4,238 2,967 0 0 8 166 77,498 6 3,338 58 49 1,213 0 wt% 0.0000 0.0473 0.0331 0.0000 0.0000 0.0001 0.0019 0.8655 0.0001 0.0373 0.0006 0.0005 0.0135 0.0000 0.0000 0.0000 Completed By: lb/hr 33 0 15,577 0 0 8 0 16 0 219,175 0 0 1 0 32,033 37,855 wt% 0.0001 0.0000 0.0511 0.0000 0.0000 0.0000 0.0000 0.0001 0.0000 0.7193 0.0000 0.0000 0.0000 0.0000 0.1051 0.1242 lb/hr 33 0 15,577 0 0 8 0 16 0 219,175 0 0 1 0 32,033 37,855 Reviewed By: wt% 0.0001 0.0000 0.0511 0.0000 0.0000 0.0000 0.0000 0.0001 0.0000 0.7193 0.0000 0.0000 0.0000 0.0000 0.1051 0.1242 EG Production 39 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description Compiled by: NPM Checked by: TAS 313 308 309 310 311 312 CO2 Stripper Feed CO2 Stripper Distillate CO2 Stripper Bottoms Stripper Bottoms from Recycle Pump Liquid from Pre-Heater CO2 Absorber Liquid Feed 304,737 214 16 164,019 27,427 160,353 0.06 25,035 221 15 214,378 0 214,378 1.00 279,702 252 16 3,699 27,673 0 0.00 279,702 252 163 3,701 27,684 0 0.00 279,702 199 156 3,575 26,743 0 0.00 291,122 194 156 3,757 28,103 0 0.00 Stream Properties Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows lb/hr 36 ACETALD 0 ARGON CARBON DIOXIDE 15,594 0 DEG 0 EG 8 EO 0 ETHANE 16 ETHYLENE 0 FORM 219,195 WATER 0 METHANE 0 NITROGEN 1 OXYGEN 0 TEG 32,033 K2CO3 37,855 KHCO3 Drexel University, CHE 483 wt% 0.0001 0.0000 0.0512 0.0000 0.0000 0.0000 0.0000 0.0001 0.0000 0.7193 0.0000 0.0000 0.0000 0.0000 0.1051 0.1242 lb/hr 36 0 15,594 0 0 8 0 16 0 9,381 0 0 1 0 wt% 0.0014 0.0000 0.6229 0.0000 0.0000 0.0003 0.0000 0.0006 0.0000 0.3747 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 lb/hr 0 0 0 0 0 0 0 0 0 209,814 0 0 0 0 32,033 37,855 wt% 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.7501 0.0000 0.0000 0.0000 0.0000 0.1145 0.1353 lb/hr 0 0 0 0 0 0 0 0 0 209,814 0 0 0 0 32,033 37,855 wt% 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.7501 0.0000 0.0000 0.0000 0.0000 0.1145 0.1353 Completed By: lb/hr 0 0 0 0 0 0 0 0 0 209,814 0 0 0 0 32,033 37,855 wt% 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.7501 0.0000 0.0000 0.0000 0.0000 0.1145 0.1353 lb/hr 0 0 0 0 0 0 0 0 0 221,235 0 0 0 0 32,033 37,855 Reviewed By: wt% 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.7599 0.0000 0.0000 0.0000 0.0000 0.1100 0.1300 EG Production 40 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description 314 315 CO2 Make-up Water After CO2 Make-up Water Feed Pump Compiled by: NPM Checked by: SSM 404 401 402 403 EO Stripper Distillate to EG Cooler EG Cooler to EG Trim Cooler EG Reactor Feed EG Reactor Product 231,563 337 132 555,422 25 555,419 0.00 lb/hr wt% 1,444 0.0062 454 0.0020 8,435 0.0364 0 0.0000 30 0.0001 81,341 0.3513 9 0.0000 6,111 0.0264 319 0.0014 133,125 0.5749 2 0.0000 7 0.0000 287 0.0012 0 0.0000 231,563 327 125 448,812 5,609 448,062 0.00 lb/hr wt% 1,444 0.0062 454 0.0020 8,435 0.0364 0 0.0000 30 0.0001 81,341 0.3513 9 0.0000 6,111 0.0264 319 0.0014 133,125 0.5749 2 0.0000 7 0.0000 287 0.0012 0 0.0000 231,542 194 118 66,312 24,251 63,070 0.00 lb/hr wt% 1,402 0.0061 473 0.0020 8,448 0.0365 0 0.0000 30 0.0001 81,342 0.3513 10 0.0000 6,111 0.0264 317 0.0014 133,106 0.5749 2 0.0000 7 0.0000 295 0.0013 0 0.0000 231,542 194 110 26,501 25,917 23,037 0.00 lb/hr wt% 1,402 0.0061 473 0.0020 8,448 0.0365 295 0.0013 109,921 0.4747 3,094 0.0134 10 0.0000 6,111 0.0264 317 0.0014 101,160 0.4369 2 0.0000 7 0.0000 295 0.0013 8 0.0000 Stream Properties Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows ACETALD ARGON CARBON DIOXIDE DEG EG EO ETHANE ETHYLENE FORM WATER METHANE NITROGEN OXYGEN TEG 11,420 88 156 182 1,364 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 11,420 1.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 Drexel University, CHE 483 11,420 86 1 182 1,362 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 11,420 1.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 Completed By: Reviewed By: EG Production 41 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description Compiled by: NPM Checked by: SSM 507 405 406 407 503 504 EG Cooler to EG Flash Drum EG Flash Vapor to Lights Compressor EG Flash Liquid to EG Dehydrator EG Dehydrator Lights to Lights Compressor Vapor Product from Lights Compressor EG Dehydrator Liquid Distillate 30,384 319 103 79,762 0 79,762 1.00 lb/hr wt% 884 0.0291 463 0.0152 8,382 0.2759 0 0.0000 630 0.0207 2,221 0.0731 10 0.0003 5,982 0.1969 211 0.0069 11,310 0.3722 2 0.0001 7 0.0002 283 0.0093 0 0.0000 201,158 319 103 3,451 25,815 0 0.00 lb/hr wt% 517 0.0026 10 0.0000 66 0.0003 295 0.0015 109,291 0.5433 873 0.0043 0 0.0000 129 0.0006 106 0.0005 89,850 0.4467 0 0.0000 0 0.0000 11 0.0001 8 0.0000 31,655 392 184 53,978 0 53,978 1.00 lb/hr wt% 1,023 0.0323 473 0.0149 8,444 0.2667 0 0.0000 630 0.0199 2,531 0.0800 10 0.0003 6,108 0.1929 233 0.0074 11,902 0.3760 2 0.0001 7 0.0002 294 0.0093 0 0.0000 90,247 192 172 1,535 11,480 0 0.00 lb/hr wt% 379 0.0042 0 0.0000 5 0.0000 0 0.0000 0 0.0000 562 0.0062 0 0.0000 3 0.0000 84 0.0009 89,213 0.9885 0 0.0000 0 0.0000 1 0.0000 0 0.0000 Stream Properties 231,542 319 103 83,213 25,815 79,762 0.14 lb/hr wt% 1,402 0.0061 ACETALD 473 0.0020 ARGON 8,448 0.0365 CARBON DIOXIDE 295 0.0013 DEG 109,921 0.4747 EG 3,094 0.0134 EO 10 0.0000 ETHANE 6,111 0.0264 ETHYLENE 317 0.0014 FORM 101,160 0.4369 WATER 2 0.0000 METHANE 7 0.0000 NITROGEN 295 0.0013 OXYGEN 8 0.0000 TEG Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows Drexel University, CHE 483 1,271 191 0 23,527 0 23,527 1.00 lb/hr wt% 139 0.1091 10 0.0075 62 0.0487 0 0.0000 0 0.0000 310 0.2441 0 0.0001 126 0.0990 22 0.0175 592 0.4657 0 0.0000 0 0.0002 11 0.0083 0 0.0000 Completed By: Reviewed By: EG Production 42 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description Compiled by: NPM Checked by: SSM 519 509 510 515 516 518 EG Dehydrator Bottoms Product Feed Pump to EG Purificaiton EG Distillate Product EG Product DEG Side Stream DEG Final Product 109,640 398 0 1,836 13,733 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 295 0.0027 109,291 0.9968 0 0.0000 0 0.0000 0 0.0000 0 0.0000 45 0.0004 0 0.0000 0 0.0000 0 0.0000 8 0.0001 109,352 270 -13 1,695 12,680 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 16 0.0001 109,291 0.9994 0 0.0000 0 0.0000 0 0.0000 0 0.0000 45 0.0004 0 0.0000 0 0.0000 0 0.0000 0 0.0000 109,352 270 0 1,695 12,681 0 0.00 lb/hr wt% 0 0.0000 0 0.0000 0 0.0000 16 0.0001 109,291 0.9994 0 0.0000 0 0.0000 0 0.0000 0 0.0000 45 0.0004 0 0.0000 0 0.0000 0 0.0000 0 0.0000 265 372 -12 4 33 0 0.00 265 372 0 4 33 0 0.00 Stream Properties 109,640 398 3 1,836 13,734 0 0.00 lb/hr wt% 0 0.0000 ACETALD 0 0.0000 ARGON 0 0.0000 CARBON DIOXIDE 295 0.0027 DEG 109,291 0.9968 EG 0 0.0000 EO 0 0.0000 ETHANE 0 0.0000 ETHYLENE 0 0.0000 FORM 45 0.0004 WATER 0 0.0000 METHANE 0 0.0000 NITROGEN 0 0.0000 OXYGEN 8 0.0001 TEG Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows Drexel University, CHE 483 Completed By: lb/hr 0 0 0 265 0 0 0 0 0 0 0 0 0 0 wt% 0.0000 0.0000 0.0000 0.9999 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0001 lb/hr 0 0 0 265 0 0 0 0 0 0 0 0 0 0 Reviewed By: wt% 0.0000 0.0000 0.0000 0.9999 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0001 EG Production 43 MASS & ENERGY BALANCE SHEET Date: 4/9/2006 Project Title: EO/EG Plant Stream Description 520 521 523 DEG/TEG Bottoms Product DEG/TEG Product EG Final Product 23 397 0 0 3 0 0.00 109,375 270 0 1,695 12,681 0 0 lb/hr wt% 0 0 0.0000 0 0.0000 30 0.0003 109,291 0.9992 0 0.0000 0 0.0000 0 0.0000 0 0.0000 45 0.0004 0 0.0000 0 0.0000 0 0.0000 8 0.0001 Compiled by: NPM Checked by: SSM Stream Properties Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows ACETALD ARGON CARBON DIOXIDE DEG EG EO ETHANE ETHYLENE FORM WATER METHANE NITROGEN OXYGEN TEG 23 397 -12 0 3 0 0.00 lb/hr Mass % 0 0.0000 0 0.0000 0 0.0000 14 0.6320 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 0 0.0000 8 0.3680 Drexel University, CHE 483 lb/hr 0 0 0 14 0 0 0 0 0 0 0 0 0 8 wt% 0.0000 0.0000 0.0000 0.6320 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.3680 Completed By: Reviewed By: EG Production 6. 45 Equipment Equipment Catalyst Item # Description R-101 Catalyst Reactor Catalyst R-401 Catalyst Reactor Catalyst Material Silver Resin Column T-201 T-202 T-301 T-302 T-501 T-502 EO Absorber EO Stripper CO2 Absorber CO2 Stripper EG Dehydrator EG Purification CL CL CL CL CS CS Compressor C-101 C-501 Recycle Compressor Lights Compressor SS SS Furnace E-601 Hot Oil Furnace CS Heat exchanger E-101 E-102 E-201 E-202A E-202B E-202C E-203 E-204A E-204B E-204C E-204D E-204E E-205 E-206 E-301 E-302 E-401 E-402A E-402B E-501A E-501B E-501C E-502A E-502B E-503 E-504 EO Reactor Pre-Heater EO Reactor Trim Cooler EO Absorber Gas Cooler EO Absorber Liquid Cooler EO Absorber Liquid Cooler EO Absorber Liquid Cooler EO Absorber Trim Cooler EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Condenser EO Stripper Re-Boiler CO2 Stripper Economizer CO2 Stripper Re-Boiler EG Reactor Pre-Cooler EG Reactor Trim Cooler EG Reactor Trim Cooler EG Dehydrator Condenser EG Dehydrator Condenser EG Dehydrator Condenser EG Dehydrator Re-Boiler EG Dehydrator Re-Boiler EG Purification Condenser EG Purification Re-Boiler Shell: SS Tube: SS Shell: CS Tube: SS Shell: SS Tube: SS Shell: SS Tube: CS Shell: SS Tube: CS Shell: SS Tube: CS Shell: CS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: CS Tube: SS Shell: CS Tube: SS Shell: SS Tube: SS Shell: SS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Packing T-201 Packing Packing T-202 Trays Tray T-301 Packing Packing T-302 Trays Tray T-501 Trays Tray T-502 Packing Packing Drexel University, CHE 483 (blank) (blank) (blank) (blank) (blank) (blank) Completed By: Reviewed By: EG Production 46 Pump P-201 P-202 P-203 P-301 P-302 P-303 P-501 P-502 P-503 P-504 P-505 P-506 P-601 P-602 EO Stripper Feed Pump EO Stripper Reflux Pump EO Absorber Recycle Pump CO2 Stripper Feed Pump CO2 Absorber Recycle Pump CO2 Absorber Make-up Pump EG Dehydrator Reflux Pump EG Purification Reflux Pump EG Purification Feed Pump MEG Product Pump DEG Product Pump DEG/TEG Product Pump Hot Oil Pump Hot Oil Pump SS SS SS SS SS SS CS CS CS CS CS CS CS CS Reactor R-101 R-401 EO Reactor EG Reactor SS SS Special X-102 X-103 (blank) Oxygen Generation Plant Oxygen Mixing Station Cooling Water System Piping CS CS (blank) SS Turbine X-101 Ethylene Feed Prep CS Vessel TK-101 TK-102 TK-103 TK-104 TK-105 V-201 V-202 V-401 V-501 V-502 V-601 MEG Storage Tank (1) MEG Storage Tank (2) MEG Storage Tank (3) MEG Storage Tank (4) DEG Storage Tank EO Absorber Flash Drum EO Stripper Reflux Drum EG Reactor Flash Drum EG Dehydrator Reflux Drum EG Purification Reflux Drum Hot Oil System (tank) CS CS CS CS CS SS SS CS SS CS SS Drexel University, CHE 483 Completed By: Reviewed By: EG Production 47 COLUMN SPECIFICATION SHEET Project Title: EO/EG Plant Item # T-201 Item Descriptioin EO Absorber Column Internal Diameter (ft) 10 Wall Thickness (in.) 0.3125 Height,T-T (ft) 50 Number of Trays 20 Tray Spacing (in.) 24 Tray Type Structured Packing Feed Tray # (from top) n/a Side Stream Plate # n/a Design Pressure (psig) 200 Material of Construction CL Fluid: Phase (V,L,V/L) V/L o Feed Temperature ( F) 176 Ovhd Temperature (oF) 248 Bttms Temperature (oF) 221 Ovhd Pressure (psig) 172 Bttms Pressure (psig) 176 Remarks: Drexel University, CHE 483 Date: 4/9/2006 Compiled by: Checked by: SKETCH Liquid Feed NPM SSM Vapor Product D =10 ft HETP = 24 in. Packing = 40 ft T-T H = 50 ft Vapor Feed Completed By: Liquid Product Reviewed By: EG Production 48 COLUMN SPECIFICATION SHEET Project Title: EO/EG Plant Item # Item Descriptioin Column Internal Diameter (ft) Wall Thickness (in.) Height,T-T (ft) Number of Trays Tray Spacing (in.) Tray Type Feed Tray # (from top) Side Stream Plate # Design Pressure (psig) Material of Construction Fluid: Phase (V,L,V/L) o Feed Temperature ( F) Ovhd Temperature (oF) Bttms Temperature (oF) Ovhd Pressure (psig) Bttms Pressure (psig) Remarks: Date: 4/9/2006 T-202 EO Stripper Drexel University, CHE 483 10 0.3125 50 20 24 Sieve 10 n/a 160 CL Compiled by: Checked by: SKETCH NPM SSM D istillate to C ondenser Reflux Return 1 Feed 10 L 194 239 194 148 162 20 D = 10 ft # of Trays = 20 Feed Tray = 10 T-T H = 50 ft Boil-up Return To Re-boiler Bottoms Product Completed By: Reviewed By: EG Production 49 COLUMN SPECIFICATION SHEET Project Title: EO/EG Plant Item # T-301 Item Descriptioin CO2 Absorber Column Internal Diameter (ft) 4 Wall Thickness (in.) 0.3125 Height,T-T (ft) 60 Number of Trays 20 Tray Spacing (in.) 24 Tray Type Structured Packing Feed Tray # (from top) n/a Side Stream Plate # n/a Design Pressure (psig) 190 Material of Construction CL Fluid: Phase (V,L,V/L) V/L o Feed Temperature ( F) 248 Ovhd Temperature (oF) 239 Bttms Temperature (oF) 194 Ovhd Pressure (psig) 162 Bttms Pressure (psig) 176 Remarks: Drexel University, CHE 483 Date: 4/9/2006 Compiled by: Checked by: SKETCH Liquid Feed NPM SSM Vapor Product D = 4 ft HETP = 24 in. Packing = 40 ft Vapor Feed Completed By: Liquid Product Reviewed By: EG Production 50 COLUMN SPECIFICATION SHEET Project Title: EO/EG Plant Item # Item Descriptioin Column Internal Diameter (ft) Wall Thickness (in.) Height,T-T (ft) Number of Trays Tray Spacing (in.) Tray Type Feed Tray # (from top) Side Stream Plate # Design Pressure (psig) Material of Construction Fluid: Phase (V,L,V/L) o Feed Temperature ( F) Ovhd Temperature (oF) Bttms Temperature (oF) Ovhd Pressure (psig) Bttms Pressure (psig) Remarks: Date: 4/9/2006 T-302 CO2 Stripper Drexel University, CHE 483 4 0.3125 40 15 24 Sieve 1 n/a 40 CL Compiled by: Checked by: SKETCH NPM SSM Distillate to Condenser Feed 1 D = 4 ft # of Trays = 15 Feed Tray = 1 T-T H = 40 ft L 214 221 252 15 17 15 Boil-up Return To R e-boiler Bottoms Product Completed By: Reviewed By: EG Production 51 COLUMN SPECIFICATION SHEET Project Title: EO/EG Plant Item # Item Descriptioin Column Internal Diameter (ft) Wall Thickness (in.) Height,T-T (ft) Number of Trays Tray Spacing (in.) Tray Type Feed Tray # (from top) Side Stream Plate # Design Pressure (psig) Material of Construction Fluid: Phase (V,L,V/L) o Feed Temperature ( F) Ovhd Temperature (oF) Bttms Temperature (oF) Ovhd Pressure (psig) Bttms Pressure (psig) Remarks: Date: 4/9/2006 T-501 EG Dehydrator Drexel University, CHE 483 10.5 0.3125 85 38 24 Sieve 15 n/a 40 CS Compiled by: Checked by: SKETCH NPM SSM Distillate to Condenser R eflux R eturn 1 Feed 15 35 L 318 192 397 0 1 D = 10.5 ft # of Trays = 38 Feed Tray = 15 T-T H = 85 ft Boil-up R eturn To R e-boiler Bottoms P roduct Completed By: Reviewed By: EG Production 52 COLUMN SPECIFICATION SHEET Project Title: EO/EG Plant Item # T-502 Item Descriptioin EG Purification Column Internal Diameter (ft) 14 Wall Thickness (in.) 0.3125 Height,T-T (ft) 80 Number of Trays 35 Tray Spacing (in.) 24 Tray Type Structured Packing Feed Tray # (from top) 14 Side Stream Plate # 26 Design Pressure (psig) 40 Material of Construction CS Fluid: Phase (V,L,V/L) L o Feed Temperature ( F) 397 Ovhd Temperature (oF) 270 Bttms Temperature (oF) 397 Ovhd Pressure (psig) -11.8 Bttms Pressure (psig) -11.5 Remarks: Drexel University, CHE 483 Date: 4/9/2006 Compiled by: Checked by: SKETCH NPM SSM Distillate to Condenser D = 14 ft R eflux Return 1 Feed HETP = 24 in. Packing = 70 ft T-T H = 80 ft Feed @ 28' Side Product @ 52' 26 35 Boil-up Return To Re-boiler Bottoms Product Completed By: Reviewed By: EG Production 53 COMPRESSOR SPECIFICATION SHEET Project Title: EO/EG Plant Item # Date: 4/9/2006 Item Description Performance Flow (cfm) Normal Flow (cfm) Design Suction Pressure (psig) Discharge Pressure (psig) TDH Normal TDH Design Hydraulic HP Number of Stages Type of Pump Speed (rpm) Material of Construction Compiled by: Checked by: C-101 Recycle Compressor NPM TAS C-501 Lights Compressor 8,600 10,750 148 221 20,000 25,000 2,920 2 Centrifugal 3600 SS 1,600 2,000 0 184 130,000 162,500 636 4 Centrifugal 3600 SS C-101 Stage Pressure Ratio Hydraulic HP Exit Pressure (psig) Exit Temperature Feed Flow (lb/hr) Total Flow (lb/hr) Electricity (kW) Cooling Water (lb/hr) Cooling (Btu/hr) 1 1 298 172 261 89,541 89,541 222 139,925 7,411,392 2 1 2,622 221 249 412,044 501,585 1,955 136,214 7,215,644 C-501 Stage Pressure Ratio Hydraulic HP Exit Pressure (psig) Exit Temperature Feed Flow (lb/hr) Total Flow (lb/hr) Electricity (kW) Cooling Water (lb/hr) Cooling (Btu/hr) 1 2 24 15 325 1,271 1,271 18 668 35,397 2 2 31 44 547 0 1,271 23 1,620 85,826 Drexel University, CHE 483 Completed By: 3 2 31 103 547 0 1,271 23 14,367 761,039 4 2 550 184 547 30,384 31,655 410 28,559 1,512,993 Reviewed By: EG Production 54 HEAT EXCHANGER SPECIFICATION SHEET Date: 4/9/2006 E-201 Project Title: EO/EG Plant Item # E-101 E-102 Item Description EO Reactor PreHeater EO Reactor Trim Cooler Compiled by: NPM Checked by: TAS E-202 EO Absorber Gas Cooler EO Absorber Liquid Cooler Exchanger Side SHELL TUBE SHELL TUBE SHELL TUBE SHELL TUBE Fluid Circulating Ethylene Vapor Mixture Ethylene Vapor Mixture Hot Oil Ethylene Vapor Mixture EO Rich Water Ethylene Vapor Mixture EO Rich Water EO Lean Water 191 392 289 86 0.01 31.2 221 191 302 73 0.02 29.8 30 302 464 741 0.93 236.4 198 482 392 87 0.01 31.2 169 248 255 193 0.47 19.2 184 294 253 108 0.01 30.9 176 220 248 195 0.59 19.2 201 260 226 198 0.89 18.3 0 0 0 0 273,231 273,231 0.50 0.31 0 0 1,207,448 1,205,139 0 0 0 0 0 601,490 601,490 0.467 Process Conditions Pressure (psig) Temperature IN (°F) Temperature Out (°F) Dew or Bubble Pts (°F) Specific gravity Molecular Weight Liquid Flows Flow IN (lb/hr) Flow OUT (lb/hr) Specific heat (Btu/lb/°F) Viscosity (cp) Vapor Flows Flow IN (lb/hr) Flow out (lb/hr) Specific heat (Btu/lb/°F) Performance Heat Duty (Btu/hr) Overall Coeff. (Btu/hr/ft²/°F) Correction Factor Log Mean ΔT (°F) Surface Area (ft²) Fouling Factor Design Number of Shells Surface Area Per Shell Tube Length (ft) Tube diameter (in.) Number of Tubes 601,490 601,490 0.437 16,907 19,216 0.326 633,146 633,146 0.401 1.06 0.26 1.14 0.22 10,567 16,907 0.321 0 0 23,601,499 50 0.9 94 5,580 0.0010 0.0010 22,131,708 50 0.9 45 10,994 0.0015 0.0010 10,013,676 50 0.9 17 13,042 0.0015 0.0010 37,158,187 150 0.9 8 32,657 0.0030 0.0030 1 5,580 20 1.00 1,066 1,066 221 246 BES 442 horiz. 1 10,994 20 1.00 2,100 2,100 198 223 BES 532 horiz. 1 13,042 20 1.00 2,491 2,491 184 209 BES 344 horiz. 3 10,886 20 1.00 6,237 2,079 201 226 BES 270 horiz. Numbe of Tubes per Shell Operating Press. (psig) Design Press. (psig) TEMA Type Design Tempe. (°F) Position(horiz./vertical) Materials of Construction 602,615 602,615 0.367 1.10 0.23 1,213,790 992,984 1,207,448 992,984 SS Drexel University, CHE 483 SS CS SS Completed By: SS SS SS Reviewed By: CS EG Production 55 HEAT EXCHANGER SPECIFICATION SHEET Date: 4/9/2006 E-205 Project Title: EO/EG Plant Item # E-203 E-204 Item Description EO Absorber Trim Cooler EO Stripper PreHeater Exchanger Side Fluid Circulating SHELL Cooling Water TUBE EO Lean Water Process Conditions Pressure (psig) 30 194 Temperature IN (°F) 86 226 Temperature Out (°F) 140 176 Dew or Bubble Pts (°F) 240 197 Specific gravity 0.98 0.91 Molecular Weight 18.0 18.3 Liquid Flows Flow IN (lb/hr) 962,730 992,984 Flow OUT (lb/hr) 962,730 992,616 Specific heat (Btu/lb/°F) 1.00 1.08 Viscosity (cp) 0.22 0.26 Vapor Flows Flow IN (lb/hr) 0 0 Flow out (lb/hr) 0 0 Specific heat (Btu/lb/°F) 0 Performance Heat Duty (Btu/hr) 50,994,691 Overall Coeff. (Btu/hr/ft²/°F) 150 Correction Factor 0.9 Log Mean ΔT (°F) 88 Surface Area (ft²) 4,297 Fouling Factor 0.0030 0.0030 Design Number of Shells 1 Surface Area Per Shell 4,297 Tube Length (ft) 20 Tube diameter (in.) 1.00 Number of Tubes 821 Numbe of Tubes per Shell 821 Operating Press. (psig) 194 Design Press. (psig) 219 TEMA Type BES Design Tempe. (°F) 136 Position(horiz./vertical) horiz. Materials of Construction CS SS Drexel University, CHE 483 SHELL TUBE Compiled by: NPM Checked by: TAS E-206 EO Stripper Condenser SHELL TUBE EO Stripper Re-Boiler SHELL TUBE EO Rick Water EO Lean Water Cooling Water EO Rich Distillate Hot Oil EO Lean Boilup Water 162 255 325 191 0.43 19.2 201 359 260 198 0.83 18.3 30 86 140 240 0.98 18.0 133 174 174 174 0.01 22.1 30 531 302 741 0.93 236.4 133 251 251 251 0.83 18.3 1,205,139 992,984 1,159,529 992,984 1.11 0.22 1.33 0.15 19,216 64,828 0.331 0 0 394,212 394,212 1.00 0.22 0 0 0 1,464,893 196,498 1,464,893 196,498 95,430 95,430 0.006016 108,947,287 150 0.9 15 52,077 0.0030 0.0030 19,308,504 50 0.9 57 7,556 0.0030 0.0010 5 10,415 20 1.00 9,946 1,989 201 226 BES 305 horiz. 1 7,556 20 1.00 1,443 1,443 133 158 BES 136 horiz. SS SS Completed By: CS 0.50 0.31 1.33 0.15 0 0 0 0 0 0 167,478,806 150 0.9 134 9,233 0.0015 1 9,233 20 1.00 1,763 1,763 133 158 BES 581 horiz. SS CS Reviewed By: SS EG Production 56 HEAT EXCHANGER SPECIFICATION SHEET Date: 4/9/2006 E-401 Project Title: EO/EG Plant Item # E-301 E-302 Item Description CO2 Stripper Economizer CO2 Stripper ReBoiler Exchanger Side Fluid Circulating SHELL CO2 Rich Water TUBE SHELL CO2 Lean Water CO2 Lean Boilup Water Process Conditions Pressure (psig) 156 163 Temperature IN (°F) 194 252 Temperature Out (°F) 236 199 Dew or Bubble Pts (°F) 186 189 Specific gravity 0.25 0.89 Molecular Weight 18.8 18.0 Liquid Flows Flow IN (lb/hr) 222,968 209,814 Flow OUT (lb/hr) 219,258 209,814 Specific heat (Btu/lb/°F) 1.05 1.13 Viscosity (cp) 0.31 0.22 Vapor Flows Flow IN (lb/hr) 11,841 0 Flow out (lb/hr) 15,551 0 Specific heat (Btu/lb/°F) 0.225 Performance Heat Duty (Btu/hr) 11,930,570 Overall Coeff. (Btu/hr/ft²/°F) 150 Correction Factor 0.9 Log Mean ΔT (°F) 10 Surface Area (ft²) 9,049 Fouling Factor 0.0030 0.0030 Design Number of Shells 1 Surface Area Per Shell 9,049 Tube Length (ft) 20 Tube diameter (in.) 1.00 Number of Tubes 1,728 Numbe of Tubes per Shell 1,728 Operating Press. (psig) 163 Design Press. (psig) 188 TEMA Type BES Design Tempe. (°F) 244 Position(horiz./vertical) horiz. Materials of Construction SS SS Drexel University, CHE 483 TUBE Compiled by: NPM Checked by: TAS E-402 EG Reactor PreCooler EG Reactor Trim Cooler SHELL TUBE SHELL TUBE Hot Oil EG Liquid Mixture EO/Water Vapor Mixture Cooling Water EO/Water Vapor Mixture 15 252 252 230 1.00 18.0 30 531 302 741 0.90 236.4 110 194 319 230 0.14 29.2 132 337 327 169 0.01 23.8 30 86 140 240 0.98 18.0 125 327 194 167 0.01 23.8 12,530 12,530 1.13 0.22 102,649 102,649 0.50 0.31 217,120 201,158 0.84 0.49 174 40,033 1.29 0.16 0 0 0 0 0 0 14,422 30,384 0.306 231,389 191,530 0.419 2,167,158 40,033 2,167,158 184,044 1.00 0.22 1.27 0.16 0 0 0 191,530 47,498 0.409 11,735,679 150 0.9 133 653 0.0030 0.0015 34,962,442 50 0.9 57 13,523 0.0030 0.0010 114,793,280 50 0.9 144 17,735 0.0030 0.0010 1 653 20 1.00 125 125 30 55 BES 302 horiz. 1 13,523 20 1.00 2,583 2,583 132 157 BES 244 horiz. 2 8,868 20 1.00 3,387 1,694 125 150 BES 136 horiz. SS CS Completed By: CS CS CS Reviewed By: CS EG Production 57 HEAT EXCHANGER SPECIFICATION SHEET Date: 4/9/2006 E-503 Project Title: EO/EG Plant Item # E-501 E-502 Item Description EG Dehydrator Condenser EG Dehydrator ReBoiler Compiled by: NPM Checked by: TAS E-504 EG Purification Condenser EG Purification ReBoiler Exchanger Side SHELL TUBE SHELL TUBE SHELL TUBE SHELL TUBE Fluid Circulating Cooling Water Water Distillate EG Boilup Liquid Hot Oil Cooling Water EG Distillate DEG Boilup Hot Oil 15 397 397 397 0.94 62.1 30 572 407 741 0.93 236.4 30 86 140 240 0.98 18.0 -13 270 270 270 1.02 62.0 -13 397 397 397 0.95 119.0 30 572 407 741 0.93 236.4 978,153 978,153 1.00 0.22 0 0 0.00 0.00 0 0 0.00 0.00 520,876 520,876 0.50 0.31 0 0 0 22,417 22,417 0.673 167,931 167,931 0.692 0 0 0 Process Conditions Pressure (psig) 30 15 Temperature IN (°F) 86 192 Temperature Out (°F) 140 192 Dew or Bubble Pts (°F) 240 230 Specific gravity 0.98 1.00 Molecular Weight 18.0 18.0 Liquid Flows Flow IN (lb/hr) 1,770,042 Flow OUT (lb/hr) 1,770,042 Specific heat (Btu/lb/°F) 1.00 Viscosity (cp) 0.22 Vapor Flows Flow IN (lb/hr) 0 6,380 Flow out (lb/hr) 0 6,380 Specific heat (Btu/lb/°F) 0 0.926512 Performance Heat Duty (Btu/hr) 93,758,223 Overall Coeff. (Btu/hr/ft²/°F) 50 Correction Factor 0.9 Log Mean ΔT (°F) 76 Surface Area (ft²) 27,479 Fouling Factor 0.0030 0.0030 Design Number of Shells 3 Surface Area Per Shell 9,160 Tube Length (ft) 20 Tube diameter (in.) 1.00 Number of Tubes 5,248 Numbe of Tubes per Shell 1,749 Operating Press. (psig) 15 Design Press. (psig) 40 TEMA Type BES Design Tempe. (°F) 136 Position(horiz./vertical) horiz. Materials of Construction CS CS Drexel University, CHE 483 237,545 1,068,533 237,545 1,068,533 0.76 0.50 0.60 0.31 0 0 0 0 0 0 88,153,978 150 0.9 58 11,327 0.0030 0.0015 52,820,275 50 0.9 155 7,551 0.0030 0.0030 42,972,272 150 0.9 58 5,522 0.0030 0.0015 2 5,664 20 1.00 2,163 1,082 30 55 BES 447 horiz. 1 7,551 20 1.00 1,442 1,442 -13 40 BES 136 horiz. 1 5,522 20 1.00 1,055 1,055 30 55 BES 447 horiz. CS CS Completed By: CS CS CS Reviewed By: CS EG Production 58 PUMP SPECIFICATION SHEET Item # P-201 P-202 Date: 4/9/2006 P-203 P-301 Item Description EO Stripper Feed EO Stripper Reflux EO Absorber CO2 Stripper Recycle Feed 318 0.85 0.17 1,141,006 345 0.83 0.15 224,402 2619 3274 132 133 100 125 96 48 60 122 142 160 200 30 Project Title: EO/EG Plant Fluid Circulating Temeprature (°F) Specific Gravity Viscosity (cp) Flow (lb/hr) Performance Flow (gpm) Normal Flow (gpm) Design Suction Pressure (psig) Discharge Pressure (psig) TDH Normal TDH Design Hydraulic HP Design Type of Pump Speed (rpm) 358 0.83 0.15 992,984 236 0.17 0.24 219,258 Compiled by: Checked by: P-302 P-303 NPM CHM P-501 CO2 Absorber Recycle CO2 Absorber Make-up EG Dehydrator Reflux 252 0.89 0.22 209,814 86 0.99 0.82 11,420 192 0.93 0.32 96,627 2335 472 457 22 2919 590 571 28 134 148 16 1 201 16 163 156 333.6348 372.4959 638.3022 605.8205 417 466 798 757 279 69 113 6 200 250 162 182 400 500 33 Centrifugal Centrifugal Centrifugal Centrifugal Centrifugal Centrifugal Centrifugal 1,800 1,800 3,600 3,600 3,600 3,600 3,600 Materials of Construction SS SS SS SS SS SS SS PUMP SPECIFICATION SHEET Project Title: EO/EG Plant Item & Flowsheet # Fluid Circulating Temeprature (°F) Specific Gravity Viscosity (cp) Flow (lb/hr) Performance Flow (gpm) Normal Flow (gpm) Design Suction Pressure (psig) Discharge Pressure (psig) TDH Normal TDH Design Hydraulic HP Design Type of Pump Speed (rpm) Date: 4/9/2006 P-504 P-505 P-502 P-503 EG Purification Reflux EG Purification Feed 270 1.02 1.17 221,169 86 0.99 0.82 109,640 270 1.02 1.17 109,352 72 90 -23 -3 225 281 42 227 283 3 0 225 281 21 209 262 -13 0 100 125 9 Compiled by: Checked by: P-506 P-601 NPM CHM P-602 DEG/TEG Product Hot Oil Pump Hot Oil Pump 372 0.96 0.90 265 397 0.95 0.84 23 302 398 0.93 0.93 0.31 0.31 1,376,000 1,781,000 1 1 -12 0 100 125 0 0 0 -12 0 100 125 0 MEG Product DEG Product 3000 3750 18 30 100 125 116 4000 5000 18 30 100 125 150 Centrifugal Centrifugal Centrifugal Centrifugal Centrifugal Centrifugal Centrifugal 3,600 3,600 3,600 3,600 3,600 3,600 3,600 Materials of Construction CS CS CS CS CS CS CS Drexel University, CHE 483 Completed By: Reviewed By: EG Production 59 REACTOR SPECIFICATION SHEET Date: 4/9/2006 R-101 EO Reactor SHELL TUBE Project Title: EO/EG Plant Item # Item Description Exchanger Side Hot Oil Ethylene Vapor Mixture Cooling Water EO Rich Water 1,103,074 30 302 540 0.93 0.50 0 602,615 213 302 572 0.013 0.407 1 1,537,523 30 86 140 1.000 1 0 47,498 118 194 194 0.055 0.306 0 Fluid Circulating Process Conditions Flow (lb/hr) Pressure (psig) Temperature IN (°F) Temperature Out (°F) Specific gravity Specific heat (Btu/lb/°F) Vapor Fraction Performance Heat Duty (Btu/hr) Compiled by: NPM Checked by: CHM R-401 EG Reactor SHELL TUBE Overall Coeff. (Btu/hr/ft²/°F) Correction Factor Log Mean ΔT (°F) Surface Area (ft²) Fouling Factor Design Reactor Type Catalyst Type Tube Length (ft) Tube diameter (in.) Number of Tubes Design Press. (psig) Design Tempe. (°F) Position(horiz./vertical) Materials of Construction Drexel University, CHE 483 131,265,859 50 0.9 32 91,157 0.0030 0.0010 83,026,236 150 0.9 78 7,894 0.0030 0.0030 Shell & Tube Silver Based 20 1.25 13,928 238 352 horiz. Shell & Tube Anion Ion Exchange Resin 20 1.25 1,206 143 244 horiz. CS SS CS SS Completed By: Reviewed By: EG Production 60 VESSEL SPECIFICATION SHEET V-201 V-202 Date: 4/9/2006 V-401 EO Absorber Flash Drum EO Stripper Reflux Drum EO Reactor Flash Drum EG Dehydrator Reflux Drum EG Purification Reflux Drum Hot Oil Storage Tank Temperature ( F) Pressure (psig) Design Conditions 325 162 345 132 319 103 192 0 270 -13 302 18 Temperature (oF) Pressure (psig) Shell I.D. (ft) Tan.-to-Tan. (ft) Wall Thick. (in.) Corrosion Allow (in.) Heads TYPE Min. Thick. (in.) Connections Nozzles, size, in. Manholes, diam.,in Materials Shell & Heads 375 187 395 157 369 128 242 40 320 40 352 43 6.7 20.0 0.3125 0.1 3.1 9.2 0.3125 0.1 5.8 17.5 0.3125 0.1 3.1 9.2 0.3125 0.1 5.0 14.6 0.3125 0.1 10.0 38.0 0.3125 0.1 Ellip. 0.3125 Ellip. 0.3125 Ellip. 0.3125 Ellip. 0.3125 Ellip. 0.3125 Ellip. 0.3125 8 18 8 18 8 18 8 18 8 18 8 18 SS SS CS SS CS SS Project Title: EO/EG Plant Item # Item Description V-501 Compiled by: Checked by: V-502 NPM SSM V-601 Operating Conditions o Drexel University, CHE 483 Completed By: Reviewed By: EG Production 7. 61 Plant Layout (Insert Plant layout 11*17) Drexel University, CHE 483 Completed By: Reviewed By: EG Production 8. 62 Operating Requirements 8.1. Utilities Table 8-1 shows the utility usage summary throughout the entire plant. The first section shows the summary on for cooling water. The plant uses 8.09 million pounds per hour of cooling water. The Largest user being the EG Reactor Trim Cooler (E-402) followed by the EG Dehydrator Condenser (E-501). Cold oil is used in the EO reactor (R-101) and the EO reactor trim cooler (E-102) to provide cooling. The hot oil can then be used to provide elsewhere in the plant to provide heat in the distillation re-boilers. The difference in energy between what is generated in the reactor and trim cooler and what is used in the re-boilers is generated in the hot oil furnace. A summary of the electricity usage is also shown along with the electricity generation in the ethylene feed prep. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 63 Table 8.1: Utility Requirement Utility Cooling Water Item # C-101 C-501 E-203 E-205 E-402 E-501 E-503 R-401 Description Recycle Compressor Lights Compressor EO Absorber Trim Cooler EO Stripper Condenser EG Reactor Trim Cooler EG Dehydrator Condenser EG Purification Condenser EG Reactor Cooling Water Sum Utility Hot Oil Use or Make Make Mass Flow (lb/hr) 276,139 2,289 962,730 394,212 2,167,158 1,770,042 978,153 1,537,523 8,088,246 Item # E-102 R-101 Description EO Reactor Trim Cooler EO Reactor E-206 E-302 E-502 E-504 EO Stripper Re-Boiler CO2 Stripper Re-Boiler EG Dehydrator Re-Boiler EG Purification Re-Boiler Make Total Use Use Total Hot Oil Sum Utility Electricity Use or Make Make Duty (Btu/hr) 14,627,036 121,223 50,994,691 19,308,504 114,793,280 93,758,223 88,153,978 83,026,236 464,783,171 Mass Flow (lb/hr) Duty (Btu/hr) 273,231 22,131,708 1,103,074 131,265,859 1,376,305 153,397,567 1,464,893 167,478,806 102,649 11,735,679 1,068,533 88,153,978 520,876 42,972,272 3,156,951 310,340,735 1,780,646 156,943,168 Item # X-101 Description Ethylene Feed Prep C-101 C-501 P-201 P-202 P-203 P-301 P-302 P-303 P-501 P-502 P-503 P-504 P-505 P-506 Recycle Compressor Lights Compressor EO Stripper Feed Pump EO Stripper Reflux Pump EO Absorber Recycle Pump CO2 Stripper Feed Pump CO2 Absorber Recycle Pump CO2 Absorber Make-up Pump EG Dehydrator Reflux Pump EG Purification Reflux Pump EG Purification Feed Pump MEG Product Pump DEG Product Pump DEG/TEG Product Pump Make Total Use Use Total Electricity Sum Drexel University, CHE 483 Completed By: Energy (kW) 175 175 2177 474 33 78 97 24 39 4 22 39 14 2 1 1 3003 2827 Reviewed By: EG Production 8.2. 64 Waste Streams Waste Streams Date: Compiled by: Project Title: EO/EG Plant 4/9/2006 Checked by: Stream # 301 309 Purge CO2 Stripper Distillate Mass Flow Rate (lb/hr) Temperature (oF) Pressure (psig) Flow Rate (cfm) Liquid Flow (gpm) Vapor Flow (cfm) Vapor Fraction Component Flows (lb/hr) 686 248 172 908 0 908 1.00 25,035 221 15 214,378 0 214,378 1.00 ACETALD ARGON CARBON DIOXIDE DEG EG EO ETHANE ETHYLENE FORM WATER METHANE NITROGEN OXYGEN TEG Total 0 28 123 0 0 0 1 516 0 8 0 0 8 0 686 Stream Description NPM SSM Stream Properties Drexel University, CHE 483 36 0 15,594 0 0 8 0 16 0 9,381 0 0 1 0 25,035 Total 36 28 15,718 0 0 8 1 532 0 9,389 0 0 9 0 25,721 Completed By: Reviewed By: EG Production 65 9. Environmental and Safety Considerations 9.1. Environmental Concerns Ethylene Oxide: Ethylene oxide rapidly breaks down when released to the environment. Because ethylene oxide is a gas, most is expected to be released to the air where it reacts with water vapor and sunlight and breaks down within a few days. Ethylene oxide will dissolve in water, but most of it will quickly evaporate to the air. The ethylene oxide remaining will be broken down by bacteria, or by reacting with water and other chemicals. When released to soil, most will evaporate to air and some may be broken down by bacteria or by reacting with water in the soil. Ethylene oxide does not persist long in the environment and is not expected to build up in the food chain. Ethylene Glycol: Ethylene glycol is a colorless, odorless, relatively non-volatile liquid. Ethylene glycol is not expected to deplete the ozone layer, it has a low potential to contribute to ground-level ozone formation, and its potential contribution to climate change is negligible. Ethylene glycol has been found to biodegrade rapidly in the aquatic environment and therefore has the potential to induce depletion of the dissolved oxygen (DO) in receiving waters. Diethlyene Glycol: DEG is a colorless, sweet smelling, relatively non-volatile liquid. DEG is readily biodegradable and breaks down when released to the environment. It is practically non-toxic to aquatic organisms on an acute basis. Triethylene Glycol: TEG is a colorless odorless clear liquid. When released into the soil, it is expected to readily biodegrade. When released into the soil, TEG is expected to leach into groundwater and is not expected to evaporate significantly. When released into water, this material is expected to readily biodegrade and not expected to evaporate significantly. TEG is not expected to significantly bioaccumulate. When released into the air, this material is expected to be readily degraded by reaction with photochemically produced hydroxyl radicals and is expected to have a half-life of less than 1 day. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 9.2. 66 Safety Concerns Ethylene Oxide : EO is very toxic and a suspected human carcinogen. Even in lower concentrations, long-term exposure of ethylene oxide leaves lasting effects on humans. The chemical is generally regarded as dangerous for the central nervous system, reproduction, genetic effects, and cancer. Laboratory research has shown the substance to increase the risk of leukemia, stomach, and brain cancer in animals. A Materials Safety Data sheet has been issued describing the health, safety and environmental properties of this product, identifying the potential hazards and giving advice on handling precautions and emergency procedures. This must be consulted and fully understood before handling storage or use. Ethylene Glycol : The major danger from ethylene glycol is from its ingestion. Due to its sweet taste, children and animals will sometimes consume large quantities of it if given access to antifreeze. Symptoms of ethylene glycol poisoning follow a three-step progression. Initially, victims may appear to be intoxicated, exhibiting symptoms such as dizziness, slurred speech, and confusion. Over time, the body metabolizes ethylene glycol into another toxin, oxalic acid. Buildup of this substance results in irregularities in the victim's heartbeat and breathing. In the final stage, the victim suffers kidney failure. In developed countries, denatonium is generally added to ethylene glycol preparations in order to offset the sweet taste. DiEthylene Glycol : DEG is harmful if swallowed. Care should therefore be exercised in all handling operations. Precautions should also be taken to prevent entry into the eyes and to prevent prolonged or repeated contact with the skin. The use of goggles or PVC or rubber gloves is recommended with additional protective clothing where necessary. Excessive exposure to mist or vapor should be minimized by provision of adequate ventilation. A Materials Safety Data sheet has been issued describing the health, safety and environmental properties of this product, identifying the potential hazards and giving advice on handling precautions and emergency procedures. This must be consulted and fully understood before handling storage or use. TriEthylene Glycol : So far there have been no adverse health effects from the inhalation or ingestion of TEG. Prolonged exposure to the skin may cause skin irritation. Splashing in eye will cause irritation with transitory disturbances of corneal epithelium. However, these effects Drexel University, CHE 483 Completed By: Reviewed By: EG Production 67 diminish and no permanent injury is expected. Vapors are non-irritating. Chronic exposure to TEG may cause possible skin irritation. Materials Safety Data sheet has been issued describing the health, safety and environmental properties of this product, identifying the potential hazards and giving advice on handling precautions and emergency procedures. This must be consulted and consulted and fully understood before handling storage or use. Leaks and Spills: In case of any leaks, we will ventilate area of leak or spill. Personal protective equipment will be used as specified in Material Safety Data Sheets. The hazard are will be isolated and unnecessary and unprotected personnel will be prohibited to enter the area. The spill will be contained and liquid will be recovered when possible. The liquid will be collected in an appropriate container or absorb with an inert material (e. g., vermiculite, dry sand, earth), and placed in a chemical waste container. Precautions will be taken so that none of the spills will be flushed down the sewers. Utilities Consideration: In case of loss of power, we will shut off the supply of ethylene, CO2 section and the purge. A back up generator will convert all the unreacted ethylene oxide into ethylene glycol after which we will shut down the plant until power is restored. Fire Prevention: Our plant will be equipped with a state of the art fire suppression/prevention system. In the event of a fire, all gas streams will be shut off. Fire fighting personnel will be required to wear a full-body encapsulating chemical resistant suit with positive pressure self-contained breathing apparatus. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 9.3. 68 Waste Minimization All the plants waste streams will be handled according to the policies and regulations of the Environmental Protection Agency (EPA). Process Recycle: A majority of the streams are recycled to optimize the process and minimize waste. The ethylene oxidation process produces a considerable amount of carbon dioxide (CO2). The waste CO2 is sent to the carbonate scrubber (T-301) and the resulting CO2 gas can be further purified and sold. Since the purification and sale of carbon dioxide is out of our scope, we have listed it as a future improvement to the overall plant, which can bring added profit to the company. For the process proposed, we will send the waste CO2 to a flare to remove the remaining hydrocarbons and impurities, releasing it to the atmosphere. In addition, our process has no waste water streams since all the water circulates in the process loop, but waste water created during cleaning will be sent to a waste water treatment plant. Finally, the bottoms product from the EG Purification Column are mixed Emissions: All waste gas steams will be sent through a flare, and emissions will be in compliance with the EPA standards. Leaks: Chemical leaks will be sent to a chemical sewer rather than a storm drain. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 69 10. Economic Feasibility 10.1. Economic Assumptions Economic Assumptions Sales: 2008 224 MM lbs 2009 448 MM lbs 896 MM lbs 2010 Ethylene Glycol Selling Price: & on 2008 38 c/lb (2 years contract) 2009 38 c/lb (2 years contract) 39 c/lb (then inc. 2%/yr) 2010 & on Variable Cost : 18 c/lb (2010 $, inflate @ 2% /yr) Fixed Cost: 23 $MM/yr (2010 $, inflate @ 2% /yr) Administration & Sales: 3 % of sales R&D: 3 % of sales Fixed Capital: 124 $MM Start - Up: Federal and State Taxes: Working Capital: Drexel University, CHE 483 15 $MM in 2007 12 % of Fixed Capital in 2007 7 $MM in 2008 6 % of Fixed Capital in 2008 5 $MM in 2009 4 % of Fixed Capital in 2009 39% 18 % of sales Completed By: Reviewed By: EG Production 70 10.2. Capital Equipment Costs Costs for capital equipment were calculated using the equipment cost spreadsheet provided to us by Arkema Chemicals Inc. The specialty equipment was priced based on the following assumptions: 1) Five miles of piping will be needed at a cost of $1MM uninstalled, with a cost factor of 5 for installation. 2) The Oxygen Mixing Station was assumed to be $1MM uninstalled, with a cost factor of 4.44 for installation. 3) The Oxygen Generation Plant was assumed to be $1MM uninstalled, with a cost factor of 5.2 for installation. Literature data found in Product and Process Design Principles by Seader, Seider and Lewin estimated capital cost for a 600 MMlb/yr ethylene oxide plant in 1995 to be $80MM. Considering increase in capacity and inflation the estimated capital costs would increase to $123MM in 2006. Our calculated capital cost is $124 MM, which is reasonable based on the above literature data. Equipment Catalyst Item # R-101 Catalyst R-401 Catalyst Description Reactor Catalyst Reactor Catalyst Material Silver Resin Capital Cost 2,274,000 9,000 2,283,000 Cost Factor 3.09 3.09 6.18 Installed Cost 7,035,000 27,000 7,062,000 T-201 T-202 T-301 T-302 T-501 T-502 EO Absorber EO Stripper CO2 Absorber CO2 Stripper EG Dehydrator EG Purification CL CL CL CL CS CS 276,000 276,000 151,000 130,000 426,000 519,000 1,778,000 5.20 5.20 5.20 5.20 5.20 5.20 31.21 1,435,000 1,435,000 785,000 676,000 2,216,000 2,699,000 9,246,000 C-101 C-501 Recycle Compressor Lights Compressor SS SS 804,000 1,048,000 1,852,000 3.48 3.48 6.95 2,795,000 3,644,000 6,439,000 Furnace Furnace Sum E-601 Hot Oil Furnace CS Heat exchanger E-101 E-102 E-201 E-202A E-202B E-202C E-203 E-204A E-204B E-204C E-204D E-204E E-205 E-206 EO Reactor Pre-Heater EO Reactor Trim Cooler EO Absorber Gas Cooler EO Absorber Liquid Cooler EO Absorber Liquid Cooler EO Absorber Liquid Cooler EO Absorber Trim Cooler EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Pre-Heater EO Stripper Condenser EO Stripper Re-Boiler Shell: SS Tube: SS Shell: CS Tube: SS Shell: SS Tube: SS Shell: SS Tube: CS Shell: SS Tube: CS Shell: SS Tube: CS Shell: CS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: SS Tube: SS Shell: CS Tube: SS Shell: CS Tube: SS Catalyst Sum Column Column Sum Compressor Compressor Sum Drexel University, CHE 483 1,800,000 1,800,000 171,000 304,000 352,000 301,000 301,000 301,000 137,000 290,000 290,000 290,000 290,000 290,000 221,000 303,000 Completed By: 4.63 4.63 4.63 4.63 5.63 6.63 4.63 4.63 5.63 6.63 7.63 8.63 4.63 4.63 Reviewed By: 791,000 1,406,000 1,628,000 1,392,000 1,693,000 1,994,000 633,000 1,341,000 1,631,000 1,921,000 2,211,000 2,501,000 1,022,000 1,402,000 EG Production Equipment 71 Item # E-301 E-302 E-401 E-402A E-402B E-501A E-501B E-501C E-502A E-502B E-503 E-504 Description CO2 Stripper Economizer CO2 Stripper Re-Boiler EG Reactor Pre-Cooler EG Reactor Trim Cooler EG Reactor Trim Cooler EG Dehydrator Condenser EG Dehydrator Condenser EG Dehydrator Condenser EG Dehydrator Re-Boiler EG Dehydrator Re-Boiler EG Purification Condenser EG Purification Re-Boiler Material Capital Cost 258,000 33,000 363,000 253,000 253,000 260,000 260,000 260,000 302,000 302,000 221,000 305,000 6,911,000 Cost Factor 4.63 4.63 4.63 4.63 5.63 4.63 5.63 6.63 4.63 5.63 4.63 4.63 138.31 Installed Cost 1,193,000 152,000 1,679,000 1,170,000 1,423,000 1,203,000 1,463,000 1,723,000 1,397,000 1,699,000 1,022,000 1,411,000 37,101,000 T-201 Packing T-202 Trays T-301 Packing T-302 Trays T-501 Trays T-502 Packing Packing Tray Packing Tray Tray Packing (blank) (blank) (blank) (blank) (blank) (blank) 454,000 177,000 72,000 58,000 285,000 1,557,000 2,603,000 3.09 4.24 3.09 4.24 4.24 3.09 22.00 1,404,000 750,000 222,000 245,000 1,208,000 4,817,000 8,646,000 P-201 P-202 P-203 P-301 P-302 P-303 P-501 P-502 P-503 P-504 P-505 P-506 P-601 P-602 EO Stripper Feed Pump SS EO Stripper Reflux Pump SS EO Absorber Recycle Pump SS CO2 Stripper Feed Pump SS CO2 Absorber Recycle Pump SS CO2 Absorber Make-up Pump SS EG Dehydrator Reflux Pump CS EG Purification Reflux Pump CS EG Purification Feed Pump CS MEG Product Pump CS DEG Product Pump CS DEG/TEG Product Pump CS Hot Oil Pump CS Hot Oil Pump CS 16,000 2,000 66,000 10,000 16,000 8,000 9,000 5,000 6,000 3,000 2,000 2,000 15,000 15,000 175,000 4.05 4.05 4.05 4.05 4.05 4.05 4.05 4.05 4.05 4.05 4.05 4.05 4.05 4.05 56.73 64,000 8,000 267,000 40,000 64,000 32,000 36,000 20,000 24,000 12,000 8,000 8,000 60,000 60,000 703,000 R-101 R-401 EO Reactor EG Reactor SS SS 259,000 230,000 489,000 5.39 5.39 10.79 1,397,000 1,240,000 2,637,000 X-102 X-103 (blank) Oxygen Generation Plant Oxygen Mixing Station Cooling Water System Piping CS CS (blank) SS 1,000,000 1,000,000 1,000,000 5,000,000 8,000,000 5.20 4.44 5.10 5.00 19.74 5,200,000 4,440,000 5,100,000 25,000,000 39,740,000 Turbine Turbine Sum X-101 Ethylene Feed Prep CS 2,245,000 2,245,000 3.48 3.48 7,812,000 7,812,000 Vessel TK-101 TK-102 TK-103 TK-104 TK-105 V-201 V-202 V-401 V-501 V-502 V-601 MEG Storage Tank (1) MEG Storage Tank (2) MEG Storage Tank (3) MEG Storage Tank (4) DEG Storage Tank EO Absorber Flash Drum EO Stripper Reflux Drum EG Reactor Flash Drum Vessel Sum 96,000 96,000 96,000 96,000 18,000 44,000 18,000 38,000 18,000 31,000 26,000 577,000 4.63 4.63 4.63 4.63 4.63 4.63 4.63 4.63 4.63 4.63 4.63 50.90 444,000 444,000 444,000 444,000 83,000 203,000 83,000 175,000 83,000 143,000 120,000 2,666,000 Grand Total 26,913,000 346.30 123,852,000 Shell: SS Tube: SS Shell: SS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Shell: CS Tube: CS Heat exchanger Sum Packing Packing Sum Pump Pump Sum Reactor Reactor Sum Special Special Sum CS CS CS CS CS SS SS CS EG Dehydrator Reflux Drum SS EG Purification Reflux Drum CS Hot Oil System (tank) SS Drexel University, CHE 483 Completed By: Reviewed By: EG Production 72 10.3. Manufacturing Costs Cost of manufacturing includes both variable and fixed costs. Variable cost includes raw materials and 20% utility costs. Fixed cost includes labor, supplies and indirect costs. Theoretical usage was calculated based on stoichiometry, while actual usage was based on our calculated annual consumption. All calculations were based on an annual capacity of 896 MMlb/yr. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 73 Product: Capacity: Location: Ethylene Glycol 896 MMlbs/ yr Gulf Cost Stream Factor: 92% (8100 hrs) Stoichiometry Ethylene Oxygen Ethylene Oxide CH2CH2 28 g/mol EO 1/2 O2 16 g/mol H2O C2H4O 44 g/mol EG C2H4O 44 g/mol EG H2O 18 g/mol H2O OHCH2CH2OH 62 g/mol DEG OHCH2CH2OH 62 g/mol H2O 18 g/mol OHCH2CH2OCH2CH2OH 106 g/mol Raw Material Ethylene Oxygen DEG (credit) Annual or Daily Comsumption (Mlb/yr) 451000 377200 2132 Utilities Fuel Power Cooling Water Average Consumption (Mlb/yr) 157 2830 8 Drexel University, CHE 483 YIELD 89% 68% Units MM btu/hr kW/yr MM lb/yr Theor. Usage lb/lb 0.45 0.26 -1.71 Price 2 0.06 10 Actual Usage lb/lb 0.51 0.38 0.00 Units $/MMBTU $/kWhrs c/1000 gal Total Delivered Price $0.35 $0.00 $0.26 Total Cost of Manuf. ($M/yr) $157,000 $0 -$554 156,000 Cost of Manuf. (c/lb) 17.68 0.00 -0.06 17.61 Cost Of Manuf. ($M/yr) 2575 1392 97 4,000 Cost of Manuf. (c/lb) 0.29 0.16 0.01 0.45 % Variable 40 20 0 20 Completed By: Reviewed By: % Variable 100 100 100 100 EG Production 74 Labor (including overhead) Operating - Board - Field Supervisor Maintenance - 2 Mechanical - 1 Instrumentational Quality Control - 2 Techs Engineer - 1 EO System - 1 CO2 System - 1 EG System - 1 OSBL Total Drexel University, CHE 483 Cost ($M/yr) Manuf. (c/lb) % Variable 500 0.06 0 250 0.03 0 800 0.09 0 15000 hrs @ $40/hr 15000 hrs @ $40/hr 1200 600 0.13 0.07 0 0 8000hrs @ $50/hr 1 per major unit(s) 1 Engineer @ $460M 1 Engineer @ $460M 1 Engineer @ $460M 1 Engineer @ $460M 800 0.09 0 460 0.05 0 460 0.05 0 460 0.05 0 460 5990 0.05 0.67 0 0 Basis 2 Operators/shift @ $250M/shift position 1 Operators/shift @ $250M/shift position 2 Operators/shift @ $400M/shift position Completed By: Reviewed By: EG Production 75 Supplies Operating Supplies Maintenance Supplies Total Supplies Indirect Costs Depreciation Taxes & Insurance Total Indirect Costs Drexel University, CHE 483 Basis 10% Operating Labor 60% Maintenance Labor Basis 11 yr. straight line 2% of Fixed Capital Cost of $M/yr Manuf c/lb % Variable 75 0.01 0 1080 1155 0.12 0.13 0 0 Cost of $M/yr 11315 2 11317 Manuf c/lb 1.26 0.00 1.26 % Variable 0 0 0 Completed By: Reviewed By: EG Production 76 10.4. Year-by-Year Economic Analysis The year-by-year analysis can be seen in the Cash Flow Model. The Internal Rate of Return is 30%, with a Hurdle Rate of 12%. Based on this data, the project is recommended to proceed further. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 77 Insert Cash flow table – 11x17 (1 pg) Drexel University, CHE 483 Completed By: Reviewed By: EG Production 78 10.5. Sensitivity & Cost Behavior Analysis Drexel University, CHE 483 Completed By: Reviewed By: EG Production 79 Ethylene Cost Sensitivity Ethylene Glycol - 896 MMlb/yr 40 35 Design Case = 30% 30 IRR, % 25 20 15 10 Hurdle Rate = 12% 5 0 0.28 0.33 0.38 0.43 0.48 0.53 Ethylene Price, $/lb Graph 1: Ethylene Cost Sensitivity The above graph represents the sensitivity of the cost of ethylene. For our base case the cost of ethylene is 35 cents/lb which yields a 30% IRR. It is apparent that the IRR follows a negative trend with increase in price of ethylene. Hence the price of ethylene should not go above 53 cents/lb in order to stay above the hurdle rate of 12%. Drexel University, CHE 483 Completed By: Reviewed By: 0.58 EG Production 80 Volume Sensitivity Ethylene Glycol - 896 MMlb/yr 35 Design Case = 30% 30 25 IRR, % 20 15 Hurdle Rate = 12% 10 5 0 -35 -30 -25 -20 -15 -10 -5 Volume, % Below Capacity Graph 2: Volume Sensitivity The above graph represents the sensitivity of the volume of Ethylene Glycol produced. For our base case the cost of ethylene is 35 cents/lb which yields a 30% IRR. The IRR decreases with cut down in our capacity. Hence our capacity should not go below 70% of the maximum capacity (896 MMlb/yr) in order for us to stay above the hurdle rate of 12%. Drexel University, CHE 483 Completed By: Reviewed By: 0 EG Production 81 Cost-Behaviour-Volume Impact Ethylene Glycol - 896 MMlb/yr 200,000 180,000 160,000 Total Cost 140,000 $M/Yr 120,000 100,000 Variable Cost = 18 c/lb 80,000 60,000 40,000 Fixed Cost = 23250 $Mlb/Yr 20,000 0 0% 10% 20% 30% 40% 50% 60% 70% 80% % Capacity Graph 3: Cost-Behavior -Volume Impact The above graph represents the cost–behavior-volume impact. As the capacity of the plant increases, the total cost, which includes the fixed and variable cost, also increases. Drexel University, CHE 483 Completed By: Reviewed By: 90% 100% EG Production 82 Break Even Chart Ethylene Glycol - 896 MMlb/yr 400,000 350,000 300,000 250,000 $M/Yr Profit Area Sales: 38 c/lb 200,000 13% 150,000 Plant Gate Cost of Sales 100,000 50,000 0 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% % Capacity Graph 4: Break Even Chart The break-even chart shows the plants break even point at 13% capacity at the selling price of 38 cents/lb of ethylene glycol. Drexel University, CHE 483 Completed By: Reviewed By: 100% EG Production 83 Price/Capacity Sensivity Ethylene Glycol - 896 MMlb/yr 60% Market Price 50% 1000 MM lb/yr Design Case = 30% 40% DCF IRR, % 896 MM lb/yr 30% 20% 600 MM lb/yr 10% Hurdle Rate = 12% 0% $0.30 $0.35 $0.40 $0.45 $0.50 Price (2006) $0.38, $/lb Graph 5: Price/Capacity Sensitivity Lowering the plant capacity by approximately 30% would cause the IRR to reach the hurdle rate at a price of 40 cents/lb. This would not be recommended. Raising the plant capacity by approximately 12% would increase our IRR for current market price. This is desirable as long as there is demand for ethylene glycol. This graph also shows the effect of change in IRR to the change in price of ethylene glycol. We can conclude that the change in capacity has a greater effect than a change in product price. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 84 Cost Behavior- Volume Impact Ethylene Glycol - 896 MMlb/yr 28.0 27.5 27.0 Operating Costs c/lb 26.5 26.0 25.5 25.0 24.5 24.0 23.5 23.0 0 10 20 30 40 50 60 70 80 90 % Capacity Graph 6: Volume impact on cost behavior As the capacity of the plant increases, the operating costs exponentially decreases. The operating cost per lb of product goes to infinity as the plant capacity goes to zero because the total product cost reaches the fixed cost. Drexel University, CHE 483 Completed By: Reviewed By: 100 EG Production 85 Cost vs Revenue 40 35 Cents/lb Product 30 25 Supplies 20 Indirect Utilities Product Labor 15 10 Raw Material 5 0 Cost Revenue Graph 7: Breakdown of Cost Factors vs. Revenue This graph compares the cost of manufacturing to the sale price of ethylene glycol. The graph shows that the raw materials contribute to majority of the cost. Because the product revenue is significantly higher than the cost of manufacturing, even with a fluctuation in raw material costs, the plant would still be profitable. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 86 11. Conclusions and Recommendations Overall, the capital investment for this project is estimated at $124 million. At an estimated production rate of 890 million pounds per year of ethylene glycol at full capacity, the plant will use 450 million pounds per year of ethylene provided by direct pipeline, and 380 million pounds per year of oxygen which will be generated by an oxygen generation plant. The anticipated Internal Rate of Return after the 16 year lifespan is expected to be 30%, with a break even period of 2 years, and this exceeds the hurdle rate of 13%. This process seams very feasible according to this preliminary economic analysis, but a few further studies can be done to improve the profitability further. Also, this process can be optimized to reduce the overall amount of heating and cooling used in the process, which could potentially save a few million dollars per year, but would probably require a small increase in the capital investment. Also, the carbon dioxide stream exiting the CO2 stripper could be purified and sold to offset the cost of production for another few million dollars savings per year. One of the most important recommendations would be to lock in the price of ethylene by entering into a long term contract with the providers. A difference of 1 c/lb in the cost of ethylene would save almost $9 million per year. At this stage, our recommendation is to proceed with this project to the next stage of development. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 12. Appendices Drexel University, CHE 483 Completed By: Reviewed By: EG Production 12.5. Sample Calculations Drexel University, CHE 483 Completed By: Reviewed By: EG Production 12.6. MSDS Drexel University, CHE 483 Completed By: Reviewed By: EG Production 12.7. Aspen Process Simulation Drexel University, CHE 483 Completed By: Reviewed By: EG Production 13. Literature Cited 1) ATSDR-ToxFAQs: Etheylen Oxide. Agency for Toxid Substances and Disease Registry. http://www.atsdr.cdc.gov/tfacts137.html 2) "BASF/ATOFINA Steam Cracker, Port Arthur, TX, USA". http://www.chemicals-technology.com/project-printable.asp?ProjectID=165 3) Bishnoi, S. 2000. Carbon dioxide absorption and solution equilibrium in piperazine activated methyldiethanolamine. The University of Texas at Austin. 4) Buckles, Carey. Chipman, Pete. Cubillas, Mary. Lakin, Mike. Slezak, Dan. Townsend, David. Vogel, Keith. Wagner, Mike. Regulations. Ethylene Oxide User's Guide. http://www.ethyleneoxide.com/html/regulations.html 5) "Carbon Dioxide Recovery and Disposal From Large Energy Systems" http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.energy.21.1.145 6) Cheminfo: Ethylene. Chemical Profiles Created by CCOHS. Canadian Centre for Occupational Health and Safety. http://www.intox.org/databand/documents/chemical/ethylene/cie89.htm 7) "Climate Information for Port Arthur, TX". http://www.rssweather.com/climate/Texas/Port%20Arthur/ 8) "Ethylene Glycol". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc, 2004. http://www.mrw.interscience.wiley.com.ezproxy.library.drexel.edu/kirk/articles/ethyfor k.a01 /pdf fs.html 9) "Ethylene Glycol". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co, 2002. http://www.mrw.interscience.wiley.com.ezproxy.library.drexel.edu/ueic/articles/a10_10 1/pdf fs.html 10) "Ethylene Glycol" http://en.wikipedia.org/wiki/Ethylene -glycol 11) "Ethylene Oxide". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc, 2004. Drexel University, CHE 483 Completed By: Reviewed By: EG Production http://www.mrw.interscience.wiley.com.ezproxy.library.drexel.edu/kirk/articles/ethydeve .a01/sect4-fs.html . 12) "Ethylene Oxide". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co, 2002. http://www.mrw.interscience.wiley.com.ezproxy.library.drexel.edu/kirk/articles/ethydeve .a01 /sect4-fs.html 13) 14} "Ethylene Oxide" http://en.wikipedia.org/wiki/Ethylene oxide "Global ethylene glycol and ethylene oxide capacity in metric tons per year for each of 58 producers, facilities or countries, reported as of March 29, 2000", Chemical Week, p 49, March 29, 2000. 15) "Hazardous Substance Fact Sheet". New Jersey Department of Health and Senior Servies. http://www.state.nj.us/health/eoh/rtkweb/0882.pdf 16) McKetta, John J. and Cunningham, William A. Encyclopedia of Chemical Processing and Design, Marcel Dekker, Inc., New York, 1983, Volume 20, pp. 282303. 17) Oxygen.-1910.104. U.S. Department of Labor. Occupational Safety & Health Administration. http://www.osha.gov/pls/oshaweb/ 18) Process Economics Program Report 2£ Ethylene Oxide and Ethylene Glycol. SRI Report, January 1997. 19) "Process for the preparation of alkylene glycols" United States Patent 5,488,184 http://patft.uspto.gov/netacgi/nphParser?Sect1=PTO 1 &Sect2=HITOFF&d=PALL&p=1 &u=/netahtml/srchnum.ht m&r=1&f=G&1=50&s1=5,488,184.WKU.&OS=PN/5,488,184& RS=PN/5,488,18 4 20) "Product Focus: EO-EG". Chemical Week, p 38, April 28, 2004. 21) Registration Eligibility Document: Ethylene. United States Environmental Protection Agency. Office of Prevention, Pesticides, and Toxic Substances. 22) Seider, Warren, Seader, J.D., Lewin, Daniel R. Product and Process Design Principles. John Wiley & Sons, Inc. 2004. Drexel University, CHE 483 Completed By: Reviewed By: EG Production 23) Synthetic Organic Heat Transfer Fluid-Liquid Phase Data: DOWTHERM RP. http://www.dow.com/heattrans/family/dowrp/index.htm?filepath=&fromPage=BasicSear ch 24) Toxicology profile for Ethylene Oxide,Agency for toxic substances and Disease Registry. http://www.atsdr.cdc.gov/toxprofiles/tp137-c4.pdf. 25) "US ethylene capacity in pounds per year by company, with plant locations". Chemical Market Reporter, p 27, September 29, 2003. Drexel University, CHE 483 Completed By: Reviewed By: 1. Introduction 2. Design Basis 3. Process Description 4. Process Flow Diagrams 5. Material & Energy Balance 6. Equipment 7. Plant Layout 8. Operating Requirements 9. Environmental and Safety Considerations 10. Economic Feasibility 11. Conclusions and Recommendations 12. Appendices 12.1 Sample Calculations 12.2 MSDS 12.3 Aspen Process Simulation 13. Literature Cited