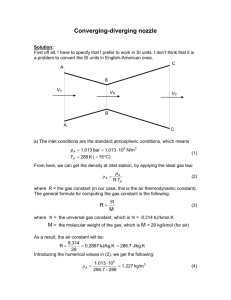
mole fractions, partial P and V
advertisement

13-12 The masses of the constituents of a gas mixture are given. The mass fractions, the mole fractions, the average molar mass, and gas constant are to be determined. Properties The molar masses of O2, N2, and CO2 are 32.0, 28.0 and 44.0 kg/kmol, respectively (Table A-1) Analysis (a) The total mass of the mixture is mm mO2 mN2 mCO2 5 kg 8 kg 10 kg 23 kg Then the mass fraction of each component becomes mf O 2 mf N 2 mf CO 2 mO 2 mm 5 kg 0.217 23 kg 8 kg 0.348 23 kg mN2 mm m CO 2 mm 5 kg O2 8 kg N2 10 kg CO2 10 kg 0.435 23 kg (b) To find the mole fractions, we need to determine the mole numbers of each component first, N O2 N N2 N CO 2 mO2 M O2 mN2 M N2 5 kg 0.156 kmol 32 kg/kmol 8 kg 0.286 kmol 28 kg/kmol m CO 2 M CO 2 10 kg 0.227 kmol 44 kg/kmol Thus, N m N O2 N N2 N CO2 0.156 kmol 0.286kmol 0.227 kmol 0.669kmol and y O2 y N2 y CO 2 N O2 Nm N N2 Nm N CO 2 Nm 0.156 kmol 0.233 0.699 kmol 0.286 kmol 0.428 0.669 kmol 0.227 kmol 0.339 0.669 kmol (c) The average molar mass and gas constant of the mixture are determined from their definitions: Mm mm 23 kg 34.4 kg/kmol N m 0.669 kmol and Rm Ru 8.314 kJ/kmol K 0.242 kJ/kg K Mm 34.4 kg/kmol 13-15E The mole numbers of the constituents of a gas mixture are given. The mass of each gas and the apparent gas constant are to be determined. Properties The molar masses of H2, and N2 are 2.0 and 28.0 lbm/lbmol, respectively (Table A-1E). Analysis The mass of each component is determined from N H 2 5 lbmol mH 2 N H 2 M H 2 5 lbmol 2.0 lbm/lbmol 10 lbm N N 2 4 lbmol m N 2 N N 2 M N 2 4 lbmol 28 lbm/lbmol 112 lbm The total mass and the total number of moles are 5 lbmol H2 4 lbmol N2 m m m H 2 m N 2 10 lbm 112 lbm 122 lbm N m N H 2 N N 2 5 lbmol 4 lbmol 9 lbmol The molar mass and the gas constant of the mixture are determined from their definitions, Mm m m 122 lbm 13. 56 lbm/lbmol N m 9 lbmol and Rm Ru 1.986 Btu/lbmol R 0.1465 Btu/lbm R Mm 13.56 lbm/lbmol 13-31 The masses of the constituents of a gas mixture at a specified pressure and temperature are given. The partial pressure of each gas and the apparent molar mass of the gas mixture are to be determined. Assumptions Under specified conditions both CO2 and CH4 can be treated as ideal gases, and the mixture as an ideal gas mixture. Properties The molar masses of CO2 and CH4 are 44.0 and 16.0 kg/kmol, respectively (Table A-1) Analysis The mole numbers of the constituents are mCO 2 1 kg mCH 4 3 kg N CO 2 N CH 4 mCO 2 MCO 2 mCH 4 MCH 4 1 kg 0.0227 kmol 44 kg / kmol 3 kg 0.1875 kmol 16 kg / kmol Nm NCO2 NCH 4 0.0227 kmol 0.1875 kmol 0.2102 kmol yCO 2 yCH 4 N CO 2 Nm N CH 4 Nm 0.0227 kmol 0108 . 0.2102 kmol 0.1875 kmol 0.892 0.2102 kmol Then the partial pressures become PCO 2 y CO 2 Pm 0.108 200 kPa 21.6 kPa PCH 4 y CH 4 Pm 0.892 200 kPa 178.4 kPa The apparent molar mass of the mixture is 1 kg CO2 3 kg CH4 300 K 200 kPa Mm mm 4 kg 19.03 kg / kmol N m 0.2102 kmol 13-31 The masses of the constituents of a gas mixture at a specified pressure and temperature are given. The partial pressure of each gas and the apparent molar mass of the gas mixture are to be determined. Assumptions Under specified conditions both CO2 and CH4 can be treated as ideal gases, and the mixture as an ideal gas mixture. Properties The molar masses of CO2 and CH4 are 44.0 and 16.0 kg/kmol, respectively (Table A-1) Analysis The mole numbers of the constituents are mCO 2 1 kg mCH 4 3 kg N CO 2 N CH 4 mCO 2 MCO 2 mCH 4 MCH 4 1 kg 0.0227 kmol 44 kg / kmol 3 kg 0.1875 kmol 16 kg / kmol Nm NCO2 NCH 4 0.0227 kmol 0.1875 kmol 0.2102 kmol yCO 2 yCH 4 N CO 2 Nm N CH 4 Nm 0.0227 kmol 0108 . 0.2102 kmol 0.1875 kmol 0.892 0.2102 kmol 1 kg CO2 3 kg CH4 300 K 200 kPa Then the partial pressures become PCO 2 y CO 2 Pm 0.108 200 kPa 21.6 kPa PCH 4 y CH 4 Pm 0.892 200 kPa 178.4 kPa The apparent molar mass of the mixture is Mm mm 4 kg 19.03 kg / kmol N m 0.2102 kmol 13-33 The masses, temperatures, and pressures of two gases contained in two tanks connected to each other are given. The valve connecting the tanks is opened and the final temperature is measured. The volume of each tank and the final pressure are to be determined. Assumptions Under specified conditions both N2 and O2 can be treated as ideal gases, and the mixture as an ideal gas mixture Properties The molar masses of N2 and O2 are 28.0 and 32.0 kg/kmol, respectively (Table A-1) Analysis The volumes of the tanks are (1 kg)(0.2968 kPa m 3 /kg K)(298 K) mRT 0.295 m 3 300 kPa P N2 V N2 (3 kg)(0.2598 kPa m 3 /kg K)(298 K) mRT 0.465 m 3 P 500 kPa O2 V O2 1 kg N2 25C 300 kPa 3 kg O2 25C 500 kPa V total V N 2 V O 2 0.295 m 3 0.465 m 3 0.76 m 3 Also, m N 2 1 kg N N 2 m O 2 3 kg N O 2 mN2 M N2 mO2 M O2 1 kg 0.03571 kmol 28 kg/kmol 3 kg 0.09375 kmol 32 kg/kmol N m N N2 N O2 0.03571 kmol 0.09375 kmol 0.1295 kmol Thus, (0.1295 kmol)(8.31 4 kPa m 3 /kmol K)(298 K) NRu T Pm 422.2 kPa 0.76 m 3 V m 13-35E A mixture is obtained by mixing two gases at constant pressure and temperature. The volume and specific volume of the mixture are to be determined. Properties The densities of two gases are given in the problem statement. Analysis The volume of constituent gas A is VA mA A 1 lbm 0.001 lbm/ft 3 1000 ft 3 and the volume of constituent gas B is VB mB B 2 lbm 0.002 lbm/ft 3 1000 ft 1 lbm gas A 3 Hence, the volume of the mixture is V V A V B 1000 1000 2000 ft 3 The specific volume of the mixture will then be v V m 2000 ft 3 666.7 ft 3 /lbm (1 2) lbm 2 lbm gas B