PART I: Energy and Mass CHAPTER 1
advertisement

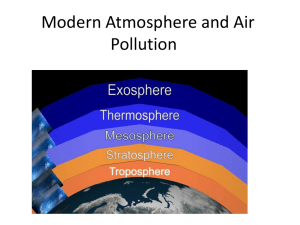
PART I: Energy and Mass CHAPTER 1 - Composition and Structure of the Atmosphere Chapter Overview: This chapter explores the thickness of Earth’s atmosphere complete with density considerations. Discussion is broken into segments, which explore the composition of the atmosphere, the role of permanent and variable gases, the origin, residence time, evolution, and the removal of each important gas. Vertical structure of the atmosphere is also described beginning with density and continuing through a temperature based profile. Chapter at a Glance: The atmosphere is a mixture of gases, small suspended solid and liquid particles, and falling precipitation. Meteorology is the study of the atmosphere and all related processes. <ME1.1> • Thickness of the Atmosphere - Although atmospheric density decreases rapidly with height, the top of the atmosphere is undefined. Overall, the majority of atmospheric mass is contained in a rather thin layer near the surface, but the atmosphere still contains an impressive sum of mass (5.14x1015kg). Due to this thinness, the majority of large-scale atmospheric motions are horizontal. But, vertical motions are exceedingly important in determining overall atmospheric behavior. • Composition of the Atmosphere - The atmosphere is an aggregate of gases, suspended, microscopic particles, and liquid water which are readily exchanged between the Earth’s surface and the atmosphere through physical (volcanic eruptions) and biological (photosynthesis, respiration) processes. Gaseous exchanges exist in a steady state to maintain concentrations. Individual gas molecules remain in the atmosphere through a relative residence time. The homosphere is an atmospheric region with chemical homogeneity, which occupies the lowest 80 km of the atmosphere. Above this, the heterosphere exists and is composed primarily of lighter gases. A. The Permanent Gases - Permanent gases such as nitrogen, oxygen, argon, and neon, occupy the homosphere. The stable gas nitrogen comprises 78% of the volume of the atmosphere yet contributes little to most meteorological and climatological processes. Diatomic oxygen dominates the remainder of the atmosphere accounting for 21% of its volume. Inert trace gases, such as argon, account for the remainder of permanent gases. B. Variable Gases - Variable gases constitute a very small portion of total atmospheric mass, however, some are extremely important with regard to atmospheric behavior, energy balance, and biological processes. 1. Water Vapor - Water vapor, the most abundant variable gas, occupying 0.25% of total atmospheric mass, is added and removed from the atmosphere through the hydrologic cycle. Concentrations near the surface exist from nearly zero, over desert and polar regions, to approximately 4% 3 near the tropics. Water vapor is a major contributor to Earth’s energy balance and many important atmospheric processes. 2. Carbon Dioxide - Carbon dioxide, a trace gas accounting for only 0.036% of the atmosphere, is an important proponent of the Earth’s energy balance. <Web> The gas is removed from the atmosphere by photosynthesis and added to the atmosphere through biologic respiration and decay, volcanic eruptions, and natural and human-related combustion. Anthropogenically related increases in recent decades have led to great concern with regard to global “greenhouse warming”. 3. Ozone - The triatomic form of oxygen is both a near surface pollutant and an essential absorber of ultraviolet radiation in the stratosphere. Ozone is formed when atomic oxygen (O) bonds with molecular oxygen (O2). <Web> <ME1.4> 4. Methane - Methane is a variable gas in small but recently increasing concentrations. It is released to the atmosphere through fossil fuel activities, livestock digestion, and agriculture cultivation (especially rice). Methane is a very effective absorber of terrestrial radiation and plays an active role in near surface warming. C. Aerosols - Any solid and/or liquid particle, other than water, which exists in the atmosphere, is known collectively as an aerosol. It is, therefore, synonymous with the term particulate. Aerosols are both natural (sea spray, dust, combustion) and human (combustion) products. Due to their small size they easily remain in suspension for long periods. As such, aerosols contribute to the precipitation process as condensation nuclei. • Vertical Structure of the Atmosphere - Distinct atmospheric layers are distinguished based on chemical composition, electrical characteristics, and temperature. A. Density - Density is mass (kg) per unit volume (m3). Due to compressibility near surface air is denser than that above so that atmospheric density decreases gradually with increasing height. This may be expressed in terms of the mean free path, or average distance a molecule travels before colliding with another. B. Layering Based on Temperature Profiles - Four distinct layers of the atmosphere emerge from identifiable temperature characteristics with height. This average temperature profile is described as the standard atmosphere. 1. The Troposphere - The lowest of all atmospheric layers is so named as this region promotes atmospheric overturning. <Web> The moniker also applies as it is in this layer that virtually all weather processes occur. Definition of the layer is marked by decreasing temperatures with height. Due to the compressibility of gases, this thinnest atmospheric layer contains 4 80% of the atmosphere’s mass. Due to thermal expansion, the top of the troposphere, the tropopause, is roughly 16 km (10 mi) over the tropics and about half that at the poles. Although temperatures fluctuate greatly through the troposphere, a steady decline with height, 6.5oC/km (3.6oF/1000 ft) occurs uniformly through the layer. Colder temperatures aloft imply that the atmosphere is essentially transparent to solar radiation and is heated through terrestrial radiation from the surface skyward. Inversions occur on rare occasions when the atmosphere warms with height. <ME1.1; 1.2, 1.3; 1.5; 1.6> 2. The Stratosphere - Little weather occurs in the stratosphere, a layer of constantly inverted temperatures. After an initial layer of constant temperature with height, the atmosphere gradually warms to near the freezing point of water at the stratopause. The inversion persists as ozone absorbs incoming ultraviolet radiation. Although the ozone layer exists through an altitude between 20-30 km (12-18 mi), actual concentrations of ozone can be as low as 10 ppm. <ME1.4> 3. The Mesosphere and Thermosphere - Combined, these two layers account for only 0.1% of total atmospheric mass. The mesosphere, which extends to about 80 km (50 mi) is characterized by decreasing temperatures with height and is the coldest atmospheric layer. Above this, the thermosphere slowly merges with interplanetary space and is characterized by increasing temperatures with height. Temperatures approach 1500oC, however, this is only a measure of molecular kinetic energy as the sparse amount of mass in this layer precludes actual heat content. • A Layer Based on its Electrical Properties: The Ionosphere - This portion of the atmosphere is replete with ions; electrically charged particles which extend from the upper mesosphere into the thermosphere. It is subdivided into the D-, E-, and F-layers with increasing height. The D and E layers diminish with reduced solar radiation allowing the F layer to reflect radio waves through the night. Interactions between the ionosphere and subatomic particles emitted from the Sun excite atmospheric gases causing the aurora borealis (northern lights) and the aurora australis (southern lights). • Evolution of the Atmosphere - The early atmosphere of the Earth was likely composed of gases such as hydrogen and helium. These light gases either exited the atmosphere to space through high escape velocities or through collisions with large celestial bodies. A secondary atmosphere formed through volcanic outgassing and by material, largely water, gained from comets. Precipitation removed, and continues to remove, excess water vapor, while high concentrations of CO2 were replaced by oxygen through photosynthesis and dissolution in water. Due to its stability, nitrogen concentrations slowly grew to present-day levels. • Some Weather Basics - Weather information has exploded in recent times. Maps, reports, etc., available only to professional meteorologists in the past are now readily available to the 5 general public via the Internet. Makes learning about weather more enjoyable since learning can be directly correlated to ongoing weather phenomena. A. Atmospheric Pressure and Wind - Pressure is one of the most fundamental weather characteristics yet it is impossible to directly sense pressure. However, pressure impacts all other aspects of weather. Air tends to blow from high pressure regions towards low pressure areas in the form of wind. Air tends to rise in low surface pressure areas and sink in high pressure areas. Rising motions favor clouds while sinking motions correspond to clear skies. Pressure is plotted on maps using isobars as units of pressure are expressed in millibars (and/or kilopascals). Station models directly depict weather station conditions at particular places. Wind speeds and wind directions are depicted by wind barbs. Wind direction is presented for the direction winds come from while the number and length of attached tick marks indicate speed. Station models also depict cloud cover with open circles indicating clear skies while closed circles depict overcast conditions. B. Temperature - An obvious weather component, which gradually changes (typically) over space. Major changes in temperature are usually associated with fronts, narrow boundaries that separate regions of warm and cold air. Four types of fronts exist, cold, warm, stationary, and occluded. C. Humidity - Relative humidity is one way, of many, to express the amount of water vapor in the atmosphere. It indicates the amount of water vapor present relative to the maximum possible. It is given as a percentage. Another index, called the dew point temperature is useful as the higher the dew point, the greater the amount of water vapor present. Dew points above 15oC (59oF) indicate humid air while dew points above 20oC (68oF) are very uncomfortable. Dew points less than about 5oC (41oF) are relative dry. Dew points are depicted at the bottom left of station models. • Planetary Atmospheres - Mercury has virtually no atmosphere due to its small size and high temperatures. The atmosphere of Venus is very thick, with a mass 90 times greater than Earth, and consists primarily of CO2 and N2. A runaway “greenhouse effect” is responsible for very high surface temperatures. The atmosphere of Mars is similar in composition to that of Venus, however, due to a low density, much terrestrial radiation is lost to space, accounting for relatively low temperatures. The Jovian planets are composed of lighter gases with either solid or liquid cores. <Web> • Weather Forecasting - Both Art and Science - Weather forecasting is highly sophisticated and data-intensive. As such, it employs state-of-the-art computers to perform millions of related calculations. Because of the complexity of information, meteorologists are rigorously trained in mathematics, physics, chemistry, and meteorology. Forecasters for the National Weather Service (NWS) and the Meteorological Service of Canada (MSC) work 24-hrs per day to create and update weather forecasts for their regions. Forecasting begins with a briefing of previous weather and current state of the atmosphere. They then examine a large amount of textual and graphic information. Computer workstations employ an Advanced Weather Interactive Processing System (AWIPS), which allows for the display of weather maps, radar images, advisories, and discussions, etc. Forecasters 6 then use a variety of methods to determine future atmospheric conditions. This information is then disseminated to the public through a variety of media. Chapter Boxes: 1-1 Focus on the Environment: Photosynthesis, Respiration, and Carbon Dioxide The basic process of plants converting water, solar energy, and carbon dioxide into simple carbohydrates is photosynthesis. Photosynthesis is part of the ongoing carbon cycle of the Earth-atmosphere system. <Web> Current global atmospheric gas concentrations are a result of this process. 1-2 Focus on the Environment: Depletion of the Ozone Layer - Chlorofluorocarbons (CFC’s), specifically chlorine atoms, react with ozone in the stratosphere. The Cl reacts with an ozone molecule to produce diatomic oxygen and chlorine monoxide. Another oxygen then interacts with the chlorine monoxide to produce diatomic oxygen and a free chlorine. Because the chlorine is unaffected in the reaction it continues to destroy ozone. In October, this process peaks over the Southern Hemisphere and persists through the spring season. The Antarctic circumpolar vortex contributes to the depletion of ozone in this area by inhibiting latitudinal mixing, thus creating an ozone hole over Antarctica through the spring season. Similar thinning of stratospheric ozone has also been found over high Northern Hemisphere latitudes. <Web> <ME1.4> 1-3 Focus on the Environment: Aerosols and Climate - Atmospheric aerosols refer to solid and liquid (other than water) particulates. Aerosol abundance plays a large role in Earth’s energy balance, typically resulting in large-scale cooling as incoming radiation is reflected back to space. Aerosols may have been instrumental in widespread extinctions as asteroids collided with the planet. Mass extinctions may have also occurred in relation to heavy volcanic activity, the so-called Pele hypothesis. 1-4 Special Interest: Recent Severe Weather - Recent (1998-99) severe weather episodes are detailed for the US. The Texas, Oklahoma, Louisiana heat wave of 1998 and the tornado outbreaks of 1998 and 1999 are highlighted. Hurricanes for both years are also described as are notable winter storms. The section closes with a discussion of extreme heat/cold events and lightning causing most weather related fatalities. <ME1.1; ME1.2> Related Web Sites: Suggested relative web sites of interest. All web sites may be obtained through www.prenhall.com/aguado by following the link for Net Search, then search by topic. Photosynthesis: www.ec.gc.ca/water/en/nature/prop/e_prop.htm Carbon Dioxide: www.co2science.org/main.htm Troposphere: www.eos.ucar.edu/eos_home.html Ozone: www.epa.gov/docs/ozone/index.html Solar System - http://pauldunn.dynip.com/solarsystem Weather: http://www.weather.com ; http://www.wrh.noaa.gov/wrhq/nwspage.html http://weatheroffice.ec.gc.ca/canada_e.html; http://www.wunderground.com Media Enrichment: 7 ME1.1 - A visible image satellite movie of the May 3-4, 1999 tornado cluster centered on Oklahoma. ME1.2 - The same images contained in ME1-1 only in enhanced IR. ME1.3 - A satellite movie depicting global 6 hour cloud images superimposed on sea surface temperatures. ME1.4 - A satellite movie detailing Antarctic Ozone Hole development during 1991. ME1.5 - The first weather satellite image of the year 2000, for the US. ME1.6 - Tropical Storm Allison Key Terms: atmosphere ozone ozone layer station model meteorology methane mesosphere front permanent gases aerosols thermosphere relative humidity variable gases particulate ionosphere dew point temperature homosphere condensation nuclei ions National Weather Service heterosphere structure aurora borealis nitrogen density aurora australis oxygen mean free path escape velocity argon standard atmosphere outgassing water vapor troposphere pressure zone forecast hydrologic cycle tropopause wind carbon dioxide inversion isobar photosynthesis stratosphere millibar respiration stratopause kilopascal Advanced Weather Interactive Processing System Meteorological Service of Canada Review Questions: 1. Why is it difficult to define an absolute top of the atmosphere? Because as the density of atmospheric gases decreases with height, the atmosphere slowly merges to space. 2. What are the homosphere and the heterosphere? Layers of the atmosphere based on the composition of gases. The homosphere describes a layer, nearer the surface, which combines all gases in proportion to the total atmospheric composition. The heterosphere lies above this region. Here, gases separate into distinct layers based on atomic weight. 3. What is the difference between the permanent and variable gases of the atmosphere? Which gases are the most important in terms of their contribution to the total mass of the atmosphere? Permanent gases are relatively stable and as such they have very long residence times. Due to long residence times permanent gases constitute the bulk of the atmosphere’s mass. Variable gases are those that are readily exchanged between the atmosphere and the surface of the Earth through various processes. They typically have relatively short 8 residence times, as Earth/atmosphere processes are adept at removing them. 4. Given that variable gases are so rare, why are they considered at all? Variable gases such as water vapor and carbon dioxide are extremely important to life functions on earth. In addition, these small percentage gases are crucial to Earth’s energy (radiation) balance. 5. Why has the concentration of carbon dioxide in the atmosphere been increasing over the last century? The principal cause for carbon dioxide increases in the atmosphere is human combustion of fossil fuels. Fossil fuels represent carbon stores over many millions of years. Human related combustion acts as a direct input of this stored carbon into the atmosphere. 6. What is ozone, and why is it both beneficial and harmful to life on earth? Life on Earth evolved without the presence of ultraviolet radiation. Ozone protects the surface from this radiation, which in high quantities is lethal to most life forms. Additionally, ozone plays an important role in Earth’s energy balance. This “good” ozone lies far above the surface in the stratosphere. Ozone itself is highly toxic. So, tropospheric ozone concentrations are considered a pollutant. 7. What are aerosols? Are they formed only be human activities or are they also naturally occurring? Solid and/or liquid particles (other than cloud drops and precipitation) suspended in the atmosphere. Aerosols have many human and natural sources. Natural sources include volcanic activity, sea spray (in the form of ejected salts), dust, pollen, and bacteria. 8. How do photosynthesis, respiration, and decay affect the carbon dioxide balance of the atmosphere? Photosynthesis and plant respiration deplete atmospheric carbon dioxide while building atmospheric oxygen, respectively. Decay of plant matter, and animal respiration adds to the total amount of atmospheric carbon dioxide. Therefore, these processes are critical to the carbon cycle and the resulting carbon balance of the atmosphere. 9. In what way does the density of the atmosphere vary with altitude? Due to the compressibility to gas, the density of the atmosphere decreases with height. 10. What are the distinguishing characteristics of the troposphere, stratosphere, mesosphere, and thermosphere? These layers are defined by their temperature characteristics with height. Distinguishing characteristics of the troposphere include an overall temperature decrease with height, the presence of virtually all of the atmosphere’s store of water vapor, and vertical mixing and 9 turbulence which contribute to general weather processes. Characteristics of the stratosphere include increasing temperatures with height due to the presence of the ozone layer. Inherent stability within this layer is also notable. The mesosphere is marked by a decrease in temperature with height, creating the atmosphere’s coldest layer. The thermosphere is characterized by increasing temperatures with height. However, this is only a function of the air’s molecular kinetic energy and not actual temperatures (due to the sparsity of gas concentrations in this layer). 11. What is the tropopause? The boundary between the troposphere below and the stratosphere above. Specifically, the tropopause represents the area where temperature stops decreasing with height. 12. In which thermal layer of the atmosphere is the ozone layer found? Why is the term “ozone layer” somewhat misleading? Ozone is found primarily in the stratosphere. The term “ozone layer” is misleading as ozone is spread throughout the stratosphere. 13. What percentage of the total mass of the atmosphere is contained in the troposphere and the stratosphere? 99.9%. 14. Why does the troposphere contain much more mass than the stratosphere, despite the fact that the troposphere is a thinner layer than the stratosphere? Gases are compressible. Therefore, the weight of the overlying atmosphere compresses the bulk (80%) of atmospheric gases in a thin layer near the surface. This represents the troposphere. 15. How is the ionosphere distinct from the layers of the atmosphere defined by their temperature profiles? The ionosphere is based on its chemistry, and not a temperature profile. The ionosphere consists of charged particles, ions, which transcend the upper reaches of the mesosphere and the thermosphere. 16. What is outgassing and why was it important? Outgassing refers to the ejection of gases from cooling molten material. Early in Earth’s history, outgassing created the atmosphere. 17. Why were anaerobic bacteria important to the evolution of the atmosphere? Anaerobic bacteria were the first respirators of oxygen. They are ultimately responsible 10 for the buildup of significant quantities of oxygen in the atmosphere, which allowed other living species to thrive. 18. Briefly describe the effect that variations in pressure exert on other weather elements. Pressure variations, caused by temperature variations, are responsible for the transport of mass in the atmosphere via wind. Pressure and wind ultimately causes many other weather phenomena such as clouds and precipitation. 19. What are isobars? Lines of equal pressure (expressed in millibar). Like all contour lines, isobars represent a three dimensional phenomena on a two-dimensional map surface. 20. What are station models and what useful information do they depict? Station models represent a useful tool, which portrays critical weather information for a location. They are included on standard weather maps and portray data on temperature, dew point temperature, wind direction, wind speed, pressure, pressure tendency, cloud cover, and precipitation. 11