- Facstaff Bucknell
advertisement

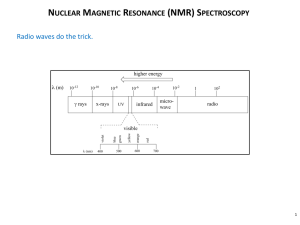
Introduction to NMR Theory in 8 Easy Steps The nuclei of some atoms have a purely quantum mechanical property called spin (a.k.a. are ‘spin-active’) and have a nonzero spin quantum number S. If a nucleus does not have spin then S=0. Sometimes one isotope of an element is spin active, while another is not. For example, the 13C nucleus has S=1/2 but the 12C nucleus has S=0. Nuclei with S=1/2, such as 1H, 13C, 15N, 31P, 19F, are most often studied in biomolecular NMR, but it is possible to take NMR spectra of nuclei that have S>1/2. Nuclei with S=1/2 can be in one of two spin states, ms=1/2 or ms =-1/2. If the nucleus does not experience a magnetic field, then the energy of the nucleus is the same no matter what the value of ms is. When a magnetic field is present, these two states have different energies as shown below. Em (ms ) (h /2 )B0 Energy s ms=-1/2 ms=1/2,-1/2 ms=1/2 Field Off Field On (B0) Notice the factors that influence the energy levels: the strength of the magnetic field (B0 which is usually in Tesla) and the gyromagnetic ratio (), which is also known as the magnetogyric ratio. The constant is specific to each nucleus: atom 1 H 13 C 15 N 31 P (107 rad T-1 s-1) 26.752 6.7283 -2.712 10.841 A nucleus can absorb energy corresponding to the difference in energy levels. We apply this energy in the form of oscillating magnetic fields. When B0=7.0 Tesla, NMR transitions range from 30 MHz for 15N to 300 MHz for 1H. NMR was initially practiced using a method called “continuous wave” or CW-NMR. This was a reasonable approach, but was slow and had poor sensitivity. Modern NMR is performed using short pulses of radio frequency magnetization, often termed pulsed Fourier-Transform NMR or FT-NMR. New alternative sampling technologies in NMR are still emerging, but FT-NMR is here to stay and will not disappear like CW NMR! 1. equilibrium magnetization in presence of external B0 2. applied oscillating magnetic field 3. splitting the oscillating field 4. the rotating frame 5. the right hand rule 6. detecting the signal 7. processing the signal 8. the return to equilibrium B0 -1/2 E = B0 = +1/2 M0 E (h / 2 )B0 First, the number of spins in the ms=1/2 and ms=-1/2 states is governed by the Boltzman equation and these levels are not equally populated in the presence of B0. A slight excess of spins precess in alignment with B0 and the sample develops a net magnetic moment M0. This is the equilibrium state. Second, we impose an oscillating magnetic field on the sample. This is accomplished by passing an oscillating current through a coil that creates an oscillating magnetic field inside the coil. The coil is wrapped around the sample. The coil is placed so that the oscillating field is perpendicular to B0 and therefore also M0. By convention, the field oscillates between –2B1 and +2B1. This will help us out soon. B0 M0 coil x 2B1sin(t) y Third, a single oscillating magnetic field of magnitude 2B1 is formally equivalent to two counterrotating fields, each of magnitude B1. One oscillating field of magnitude 2B1 Two counter-rotating fields of magnitude B1 each. Fourth, choose the one rotating field that moves in the same direction as the Larmor precession, and jump onto it! Imagine sitting on the rim of a spinning record. To you, the record looks still since you are moving with it. Now “sit” on one of the rotating components: it looks static, and the other M0 M0 component appears to be moving twice as fast. From now on we ignore the B1 B1 other component. This is the rotating frame. LAB Frame Rotating Frame Fifth, the B1 field exerts a force on M0, (torque) and causes M0 to change orientation. Point the fingers of your right hand along the applied field B1. Curl your fingers towards M0. Your thumb points in the direction z of motion for M0. In fact, M0 will rotate around in the x-z plane until B1 is M0 turned off! (B0 is not drawn in the rotating frame for reasons that can be skipped here). A 90 degree pulse : if we turn off the B1 field exactly when M0 has rotated by 90o and is aligned with the x axis, then we have applied a 90 degree pulse. A 180 degree pulse : iturn off the B1 field at exactly the time when M0 has revolved to point along the –z axis. y B1 x B1 along y rotates M0 in the x-z plane Sixth, following a 90o pulse, the B1 field is off and the sample only sees the static field B0. The right hand rule again tells us that M0 will rotate around and around in the x-y plane. This will occur at the Larmor frequency plus the chemical shift. z B0 ... and induces an oscillating current in the coil that is digitized by a computer V tim M x M0 rotates in the x-y plane Seventh, the time-domain oscillation occurs at the NMR frequency (the base Larmor frequency plus the chemical shift). It is not easy to interpret the data in this form! To see the data as a histogram of frequencies present and their relative proportions, perform a fast-Fourier transform (FFT). A method called ‘quadrature detection’ is used to determine if each NMR signal appears at + or –. analog signal is digitized FFT, Quadrature To illustrate the importance of chemical shift, recall the example of ethanol, which exhibits three signal regions for: methyl, methylene, and hydroxyl hydrogen nuclei. The signal at 0 ppm is due to tetramethylsilane (TMS) whose proton frequency is defined to be 0 ppm. The signal at about 4.7 ppm could be the –OH signal but could also be residual water in the sample. The convention for reporting NMR frequencies is the ppm scale, and is based on the frequency of a bare nucleus. If a bare proton nucleus resonates at exactly 500 MHz, then 1 ppm = 500 Hz on the proton scale. Frequencies are reported as a shift relative to a defined 0 point. A nucleus gives the same shift in ppm units no matter what field is used. (e.g. the methyl triple is at 1.1 ppm at any field strength) The ethanol spectrum shows split resonances. This effect is also called the scalar or J coupling and is a through-bond effect. Splitting of NMR signals tells a lot about the local bonded environment of a molecule. It is common to prepare proteins isotopically enriched in 15N, coupled spectrum and/or 13C. In these cases the 13C-1H or 1H-15N J couplings can arise and a great deal of effort is devoted to collapsing such heteronuclear splittings using a method called decoupling. We will not describe the decoupled spectrum specifics of how to carry out decoupling; but the result should be a A cartoon of the collapse of J-doublets upon collapse of multiplets to single lines. decoupling. Doublets are common in 1H(X) situations such as 1H-15N. Eighth, a sample of spins will return to the initial equilibrium state, given enough time. This is relaxation. The relaxation of NMR signals for macromolecules is very sensitive to the shape and size of the molecule and to all of the possible motions of the molecule. There are two fundamental ways that we view relaxation: (i) the disappearance of M0 in the x-y plane (R2 relaxation) and (ii) the reappearance of M0 along z (R1 relaxation) A key feature of macromolecules is that R2 is faster than R1, as shown below. The symbol R represents the rate of relaxation. Longitudinal (R1) Relaxation y x Transverse (R2) Relaxation y x time M0