Lecture notes on chemical properties of soil
advertisement
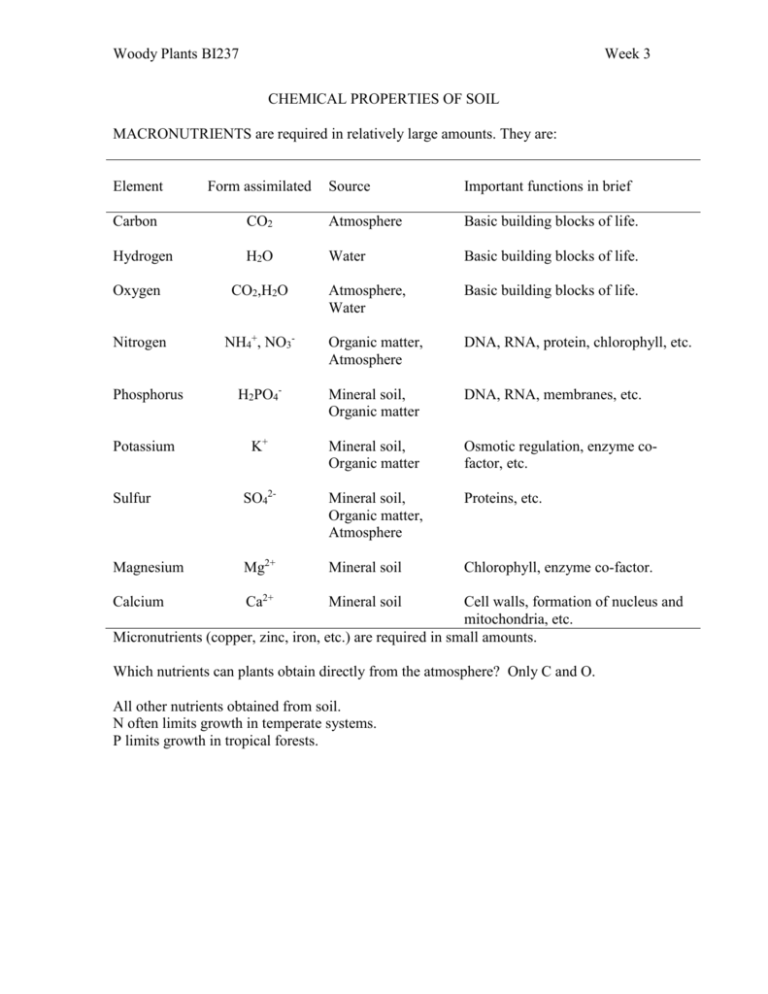
Woody Plants BI237 Week 3 CHEMICAL PROPERTIES OF SOIL MACRONUTRIENTS are required in relatively large amounts. They are: Element Form assimilated Source Important functions in brief Carbon CO2 Atmosphere Basic building blocks of life. Hydrogen H2O Water Basic building blocks of life. Atmosphere, Water Basic building blocks of life. Oxygen CO2,H2O Nitrogen NH4+, NO3- Organic matter, Atmosphere DNA, RNA, protein, chlorophyll, etc. Phosphorus H2PO4- Mineral soil, Organic matter DNA, RNA, membranes, etc. Potassium K+ Mineral soil, Organic matter Osmotic regulation, enzyme cofactor, etc. Sulfur SO42- Mineral soil, Organic matter, Atmosphere Proteins, etc. Magnesium Mg2+ Mineral soil Chlorophyll, enzyme co-factor. Calcium Ca2+ Mineral soil Cell walls, formation of nucleus and mitochondria, etc. Micronutrients (copper, zinc, iron, etc.) are required in small amounts. Which nutrients can plants obtain directly from the atmosphere? Only C and O. All other nutrients obtained from soil. N often limits growth in temperate systems. P limits growth in tropical forests. Woody Plants BI237 Week 3 CATION EXCHANGE CAPACITY Notice that many macronutrients have a positive charge (NH4+, K+, Mg2+, Ca2+) (Positively charged ions are called cations, negatively charged ions are called anions.) Clay micelles (phyllosilicates -- like layers of pages or leaves) are negatively charged. Opposite charges attract, therefore soil will hold nutrient cations in solution. The Cation Exchange Capacity (CEC) represents the total amount of cations (in centimoles) that can be adsorbed by a kilogram of soil. For example, a moraine with sugar maple and basswood may have a CEC of 18 centimoles/kg, while an outwash plain with oaks and pines may have a CEC of 12 centimoles/kg. When cations in the soil are adsorbed by plants, those held by clay particles are released. A new equilibrium is established. Adsorbed cations are a "reservoir" of nutrients. Some cations are held onto the clay very strongly. They are not so available to plants. Some are less attracted to the clay. They are easier for plants to obtain but are also more easily lost from the soil by leaching. (With the exception of hydrogen, larger ions and those with a large positive charge are most strongly held.) Here they are in order, from most to least tightly held: Al3+ > H++ > Ca2+ > Mg2+ > K+ = NH4+ > Na+ Cation exchange capacity rises as the proportion of clay rises. However, highly weathered clays like those in old tropical soils have lower CEC. ============================================================== SOIL ACIDITY pH = -log [H+] pH 7 is neutral. Below that, each value on the scale has 10X more H+ ions in solution. pH strongly influences nutrient availability. If there are lots of H+ ions in the soil, they will tend to pre-empt the other cations from sticking to the clay. (Remember that clay has a higher affinity for H+ than it does for almost anything else.) This can increase rates of mineralization and decrease activity levels of some soil organisms. An important source of H+ is respiration of roots and soil micro-organisms. CO2 + H2O --> H2CO3 This weak acid (carbonic acid) can dissociate, releasing H+: H2CO3 --> H+ + HCO3 - Woody Plants BI237 Week 3 Conifer needles release even stronger organic acids that can hasten the acidifying of forest soils. Human industrial activity has also increased soil acidity (from acid rain). Soils that are already acidic are especially vulnerable. =============================================================== SOIL ORGANIC MATTER Helps soil "hang together" (improves structure) Holds water and nutrients Supports microbes -- these are critically important in nutrient cycling What is it made of? Mostly cellulose, hemicellulose, and lignin -- materials in cell walls of plants When broken down by micro-orgs, get: CO2 and humus What is humus? Chemically-resistant; dark in color, can remain for hundreds of years Often has a negative charge -- can hold lots of nutrient cations =============================================================== HOW DO NUTRIENTS GET INTO THE SOIL IN THE FIRST PLACE? (Decomposition) Weather from minerals (but not N) Fall from sky (especially nitrates and sulfates from pollution) Micro-organisms "fix" atmospheric N. Remember that N is often a limiting factor for plants in temperate zones. Even though N2 is the most common gas in the atmosphere, higher plants can't use it from there. Micro-orgs transform it into NH4+, a biologically useful form. Fixation requires energy. Woody Plants BI237 Week 3 WHO FIXES NITROGEN? 1. Free-living N-fixing saprophytic bacteria These organisms get the energy they need from decomposing organic matter. They are restricted to ecosystems, like the Pacific Northwest and Northeastern US, where there is a lot of organic matter lying around. They don't really contribute much to the N budget. 2. Photosynthetic bacteria (aka cyanobacteria) Cyanobacteria photosynthesize to get the E they need. They need lots of light. Sometimes they get together with fungi (the partnership is called a lichen). Nostoc is a cyanobacteria inside lichens that grow on crowns of old-growth Douglas-fir. 3. Symbiotic bacteria live inside plant roots; induce formation of nodules, where N fixed. Get lots of E from the plant; supply lots of N in return A. Rhizobium -- bacterium that "infects" many members of the legume family Includes many trees in the New World tropics. Black locust. B. Frankia are actinomycetes (filamentous bacteria). Live with Alnus, Comptonia, and bayberry among others.









