PHYS2810 Atmospheric Physics
advertisement

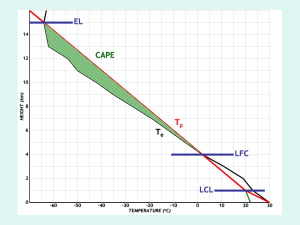
PHYS2810 Atmospheric Physics PHYS2801 Atmospheric Science Questions and Problems: Thermodynamics 1. The Earth has a radius of 6370 km. At the surface, the average atmospheric pressure is 1013.25 hPa (ignoring mountain ranges). Compute the air mass above each 1m2, and also the total mass of the atmosphere. (Assume that g = 9.8 ms-2 and is constant.) 2. Use the ideal gas equation to compute the air density at the top of Mt. Everest, where the pressure is 310 hPa and the temperature is –40C. 3. The “average” mass of an air molecule is 29 in standard units. Determine the number of molecules per cubic metre at ground level, where the pressure is 1000 hPa, and the temperature is 20 C. (You may need to look up some information.) 4. If the tropopause is at 150 hPa, and the stratopause is at 1 hPa, calculate the total mass, per unit area, of the stratosphere. How thick would the stratosphere be, if brought to STP (273 K and 1013 hPa). Take g = 9.7 ms-2. 5. Consider a constant density model of an atmosphere: that is, assume that the density is everywhere constant at 1.25 kgm-3. How thick would such an atmosphere be to account for the observed surface pressure? Use the ideal gas equation, and the hydrostatic equation, to determine the temperature profile of such an atmosphere. In particular, find both the lapse rate, , and the temperature at the top and bottom of this atmosphere. 6. Assume that the density of the atmosphere decreases exponentially with height from its surface value (1.25 kgm-3). Calculate the scale height which is consistent with the observed surface pressure. 7. Obtain the relationship between pressure, p, and height, z, in terms of the surface values of pressure and temperature, under the assumption that the temperature decreases uniformly with height at a rate /km. 8. Calculate the scale height of the Martian atmosphere, assuming a temperature of 220 K, a gravitational acceleration of 3.8 ms-2, and the fact that it is mainly composed of carbon dioxide. Also find the gas constant for such an atmosphere. 9. In the winter hemisphere, the 500 hPa level is usually at a height of 5900 m at latitude 30, and at a height of 5500 m at latitude 70. What is the mean temperature of the layer between 1000 hPa (surface) and 500 hPa in each case? 10. Assume that the average temperature between the 1000 hPa and 900 hPa pressure levels is 5C. What is the physical thickness of this layer? 11. Calculate the work done in isothermally compressing 2 kg of dry air to one fifth of its volume, at 15C. 12. 1 kg of air, at a temperature of 17C, rises from an initial pressure level of 1000 hPa, to a final pressure level of 750 hPa. (a) If the process is isothermal, calculate the work done, and the amount of heat added in the process. (b) If the process is adiabatic, calculate the work done, and determine the final temperature of the air. 13. During a cloudless night, the ground surface may lose radiant heat at the rate of 50 Wm -2 for 8 hours. Assume that all this heat is drawn from the lowest 30 hPa of the atmosphere. Find the resulting fall in the temperature of this layer of air. Repeat this calculation if some of the heat is drawn from the top 20 cm of the ground. (Assume a density of 2000 kgm-3, and a specific heat of 2000 J/kg/K.) 14. A commercial airliner suffered a sudden de-pressurization due to the loss of a cargo door. If the internal and external pressures were 850 and 350 hPa respectively, and the internal temperature was 22 C before de-pressurization, determine the final internal temperature. (Hint: what can you assume about the process?) 15. Surface winds blow down a mountain range during a Chinook. If the temperature at 14,000 ft (4200 m) is -10C, what is the surface temperature in Denver, at an elevation of 5000 ft (1500 m)? 16. A plume of heated air leaves the cooling tower of a power plant with a temperature of 30C. To what height will the plume ascend (i.e. at what height will it be in equilibrium with the surrounding air) if the ambient temperature varies with altitude according to (a) T(z) = 20 – 8z, and (b) T(z) = 20 +z ? (T in C, z in km). 17. Use the F160 chart to determine the following: Given a) T = 0C, p = 700 hPa b) T = 20C, p = 1050 hPa c) T = -20C, p = 400 hPa d) = 40C, p = 400 hPa e) = -20C, p = 900 hPa f) = 40C, p = 800 hPa g) = 40C, T = 0C h) = 60C, T = -20C Find (C) (C) (C) T (C) T (C) T (C) p (hPa) p (hPa) 18. Calculate the virtual temperature of moist air which has a temperature of 32C, and a water vapour mixing ratio of 15 g/kg. 19. 20 litres of moist air at 20C, with a relative humidity of 60%, are compressed isothermally to a volume of 4 litres. Calculate the mass of water condensed. (You will need to consult a table of saturation vapour pressures.) 20. Use the Clausius-Clapeyron equation to estimate the saturation vapour pressure at 20C, and at 35C, and compare your answers with tabulated values. Also determine the saturation vapour pressure at -20C over both ice and liquid water. 21. The Clausius-Clapeyron equation may be used to determine the boiling point of water, defined as that temperature at which saturation vapour pressure equals ambient pressure. Determine the boiling point at standard pressure (1013.25 hPa), and also in Denver, and on top of Mt. Everest. 22. An approximate expression for boiling point as a function of elevation was given in the notes. Derive it by assuming that pressure decreases exponentially, p p0 exp z / H , combining this with equation (2.6), and remembering that water boils when es = p. Find the value of the constant a. What boiling points does this formula predict at sea level, and also at Denver? (Assume the scale height, H = 8.5 km.) 23. At a pressure of 1000 hPa find r in the following situations (use the F160) a) T = 10C, RH = 100% b) T = 20C, RH = 20% c) T = 5C, RH = 50% d) e) f) g) h) T = 10C, RH = 20% T = 20C, RH = 50% T = 5C, RH = 100% T = 40C, RH = 100% T = 40C, RH = 2% 24. Repeat Question (21) for a pressure of 850 hPa. 25. Air at 1000 hPa and 25C has a wet-bulb temperature of 20C. Find the dew point temperature. If this air were expanded until all the moisture condensed and fell out, and then re-compressed to 1000 hPa, what would be the resulting temperature? 26. An air parcel at 1000 hPa has an initial temperature of 15C and a dew point of 4C. Find the mixing ratio, relative humidity, lifting condensation level, wet-bulb temperature and equivalent potential temperature of the air. 27. Air at a temperature of 20C, and a mixing ratio of 10 g/kg, is lifted from 1000 hPa to 700 hPa in moving over a mountain. What is the initial dew point of the air? Assume that 80% of the condensed water vapour is removed by precipitation during ascent. Determine the air temperature after it has descended to 900 hPa on the lee side. 28. Plot the following sounding: Pressure (hPa) Temperature (C) Dew Point (C) A 1000 30.0 21.5 B 970 25.0 21.0 C 900 18.5 18.5 D 850 16.5 16.5 E 800 20.0 5.0 F 700 11.0 4.0 G 500 13.0 20.0 State whether the 6 layers, AB, BC, etc are in stable, unstable or neutral equilibrium. State which layers are convectively unstable. 29. Aerosol particles with radii between 1 and 20 m experience a Stokes drag force which is given by 6rv, where = 1.7 x 10-5 Nsm-2 is the viscosity of air, and v is the particle’s velocity. Derive a general expression for their fall speed through air as a function of radius, and use it to determine the fall speeds of aerosols with radii of 1 and 10 m. (Assume their density is 2 x 103 kg/m3.) 30. A drop with an initial radius of 100 m falls through a cloud containing 100 droplets per cubic centimetre, which it collects in a continuous manner with a collection efficiency of 0.8. If all these droplets have a radius of 10 m, how long will it take for the drop to reach a radius of 1 mm? Assume that the smaller droplets are stationary, and that the larger droplet’s terminal velocity, V (in metres per second), is related to its radius, R (in metres), by V = 6 x 103 R. 31. A cloud which is cylindrical in shape has a cross-sectional area of 10 sq. km, and a height of 3 km. The whole volume of the cloud is initially supercooled, and the liquid water content is 2 g/m3. If all of this water is transferred onto ice nuclei, present in a uniform concentration of 1 per litre, calculate the mass of each crystal produced, and the total number of ice crystals in the cloud. If all the ice crystals precipitate, and melt before they reach the ground, what will be the total rainfall produced? Questions and Problems: Dynamics 1. Why does air pressure decrease with height more rapidly in cold air than in warm air? 2. On an upper level chart, is cold air generally associated with low or high pressure? What about warm air? 3. Explain why, in the Southern Hemisphere, the average height of contour lines on an upper-level isobaric chart tend to decrease southward. 4. Why would you not expect to observe a geostrophic wind at the equator? 5. Describe how the wind blows around highs and lows aloft and near the surface (a) in the Northern Hemisphere and (b) in the Southern Hemisphere. 6. Why are highs associated with ridges and lows associated with troughs on the 500 hPa surface? 7. What factors influence the angle at which surface winds cross the isobars? 8. Describe the type of vertical motions associated with high and low pressure areas. 9. Suppose an aircraft using a pressure altimeter flies along a constant pressure surface from standard temperature into warmer-than-standard air without any corrections. Would the altimeter indicate an altitude higher or lower than the aircraft’s true altitude? Explain. 10. What differences might you expect to see between the weather conditions on the surface chart for Sydney, and at the 500 hPa level above us. 11. Explain how and why the average surface pressure features shift from summer to winter. 12. Why is it impossible on the Earth for a Hadley cell to extend from the equator to the pole? 13. What features of the Earth’s general circulation help to determine where the driest and wettest places are found? 14. Over the open ocean in the vicinity of the Pacific high, observations have indicated that ozone concentrations hundreds of metres above the surface are greater than expected. Give a possible explanation for this. 15. Give two reasons why pilots would prefer to fly in the core of a jet stream rather than just above or below it. 16. How does the polar front influence the development of the polar-front jet stream? 17. Why is the polar-front jet stream stronger in winter than in summer? 18. What is a major El Nino event? What happens to the surface pressure at opposite sides of the Pacific Ocean during the Southern Oscillation? 19. What type of weather (cold/warm, wet/dry) would you expect over Australia during a strong El Nino? During a strong La Nina? 20. The Coriolis force deflects moving water to the right in the northern hemisphere, and to the left in the southern hemisphere. Why then does upwelling tend to occur along the western margin of continents in both hemisphere? 21. Determine the speed of the geostrophic wind near the surface, where the air density is 1.0 kg/m3, at latitudes of 10, 30 and 60, for a pressure gradient of 5 hPa/100 km. Answers: Thermodynamics 1. 10339 kg; 5.272 x 1018 kg. 2. 0.46 kg/m3. 3. 2.47 x 1025 molecules m-3. 4. 1536 kg/m2; 1188 m. 5. 8.27 km. 6. 8.27 km. 7. T z 0 R / g p 1 p0 8. 10.94 km; 189. 9. 17.65C; -2C. 10. 858 m. 11. 266 kJ. 12. 23.9 kJ, 23.9 kJ; 16.5 kJ, -6C. 13. 4.7C; 1.3C. 14. -44C. 15. 16.5C. 16. 5.555 km, 0.926 km. 17. 29C; 16C; 57C; -32.5C; -28C; 20.5C; 620 hPa; 385 hPa. 18. 34.8C. 19. 0.138 g. 20. 23.67 hPa, 58.25 hPa, 1.27 hPa, 1.03 hPa. 21. 94.7C, 90.4C, 67.4C. 22. 14.73 23. 1.8, 3.0, 2.7, 0.36, 7.5, 5.4, 49, 1.0 (g/kg). 24. 2.2, 3.5, 3.3, 0.44, 8.75, 6.6, 59, 1.2 (g/kg). 25. 18C, 60C. 26. 5.1 g/kg, 47%, 850 hPa, 9C, 28C. 27. 13C, 21C. 28. 29. 2.56 x 10-4 m/s, 2.56 x 10-2 m/s. 30. 4600 seconds. 31. 2 mg, 3 x 1013, 6 mm. Dynamics 21. 198 m/s, 69 m/s, 40 m/s.