CHAPTER 10 - NUCLEAR PHYSICS
advertisement

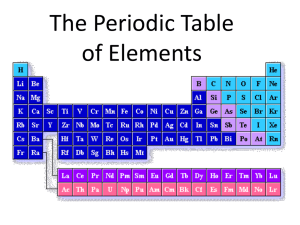
CHAPTER 11 - CHEMICAL ELEMENTS Chemistry is the branch of physical science that deals with the composition and structure of matter and the reactions that occur which change one substance into another. An early form of chemistry called alchemy flourished from about 500 AD to 1600 AD. Its main goals were to find ways to change base metals into gold and to discover an "elixir of life" that would prevent aging. Modern chemistry began in the 1700's with the work of Lavoisier. It uses logic in an attempt to explain changes in matter and avoids superstition, mysticism, and secrecy. There are 5 major divisions of chemistry. 1. Physical chemistry - applies the theories of physics to the study of chemical systems. 2. Analytical chemistry - the study of determining what substances and how much of each are present in a material. 3. Organic chemistry - the study of compounds that contain carbon. 4. Inorganic chemistry - the study of all other chemical compounds. 5. Biochemistry - the study of chemical compounds and chemical reactions that occur in living things. Classification of Matter Figure 11.1 A Chemical Classification of Matter Copyright © Houghton Mifflin Company 11-2 Matter is anything that has mass. Any form of energy has no mass and is not matter. A pure substance is a type of matter in which all samples have the same composition and the same properties. Only elements and compounds are considered pure substances. An element is a substance in which all of the atoms have the same number of protons. Example Hydrogen - H, Oxygen - O, Sodium - Na, Chlorine - Cl A compound is a substance composed of two or more elements chemically bound in a definite fixed ratio by mass. Example Water - H2O, Sodium Chloride NaCl A compound can be broken down into its component elements by chemical means. An element cannot be broken down by chemical means. Only a nuclear reaction can change an element into a different element. A mixture is a type of matter composed of two or more substances that are physically mixed, not chemically bonded. Different samples of a mixture may have different compositions and properties. A heterogeneous mixture is one that is not uniform. Different substances can be observed. Examples Pizza, Italian salad dressing, Chocolate chip ice cream A homogeneous mixture is composed of two or more substances that are mixed so thoroughly that they cannot be distinguished. A solution is a type of homogeneous mixture. Examples Coffee, Air, Brass In a solution, the substance present in the larger amount is called the solvent. The substance that has been dissolved is called the solute. When water is the solvent, the solution is said to be aqueous. When a solution contains all of the solute it can hold, it is said to be saturated. If more solute can be dissolved in it it is unsaturated. If the solution contains more solute than it normally could dissolve at that temperature, it is said to be supersaturated. Solubility of a solute is the amount of a solute that can be dissolved in a specified mass or volume of solvent at a specific temperature. In the case of liquid solvents and solid solutes, solubility increases as the temperature increases. In the case of liquid solvents and gaseous solutes, the solubility decreases as the temperature increases. Figure 11.5 The Effect of Temperature on Solubilities of Salts in Water Molecules and Allotropes A molecule is an electrically neutral particle composed of two or more atoms chemically bonded. If all the atoms are from the same element, it is a molecule of an element (H2, N2, O2, O3). If the atoms are from two or more different elements, it is a molecule of a compound. Figure 11.11 Representations of Molecules Copyright © Houghton Mifflin Company 11-5 Allotropes of an element exist when atoms of the element form different types of bonds and different physical structures. An example is carbon with diamonds, graphite and fullerenes (Bucky balls). The Periodic Table The periodic table is an organizational chart of the elements. They are arranged in order of increasing atomic number, horizontal rows called periods and vertical columns called groups. The properties of the elements are relatively constant within a group but vary across a period in a repeating fashion. The periodic law states: The properties of elements are periodic functions of their atomic numbers. Dmitri Mendeleev developed the first useful periodic table. Its modern form is still in use today. Figure 11.16 Names of Specific Portions of the Periodic Table The elements are grouped in several ways. In one, the representative elements are those in groups 1A through 8A. These elements contain 1 to 8 electrons in the outer shell and contain the specially named groups alkali metals (group 1A), alkaline earth metals(group 2A), Halogens(group 7A), and the Noble gasses(group 8A). In this classification method, the elements in groups 3B through 12B are called the transition elements and contain metals like copper, silver and gold. They are between groups 2A and 3A. The inner transition elements are the two rows called the Lanthanides and Actinides and are placed in a separate area at the bottom of the chart. Uranium and plutonium belong in this classification. Another way to classify elements is into the categories of metals and nonmetals. Elements on the left side of the chart including all of the transition elements and inner transition elements are metals. The elements in the upper right hand corner and far right on the table are called nonmetals. The elements that form a zigzag line between the metals and nonmetals are called metalloids. Properties of Metals Good conductors of heat and electricity Malleable - can be beaten into thin sheets Ductile - can be stretched into wire Possess metallic luster Solid at room temperature(except Hg) Have 1 to 3 valence electrons Lose electrons to form positive ions Properties of Nonmetals Poor conductors of heat and electricity Brittle if solid Nonductile No metallic luster Solid, liquid, or gas at room temperature Have 4 to 8 valence electrons Gain electrons or share electrons Elements near the bottom of the chart have stronger metallic characteristics. Elements to the right on the chart have stronger nonmetal characteristics. The electrons in an atom are arranged in major energy levels called shells. On the periodic table each period corresponds to the beginning of a shell. The shells are further subdivided into sub shells which are composed of orbitals each with one or two electrons with different spins. Figure 11.18 Shell Distribution of Electrons in Periods 1, 2, and 3 Copyright © Houghton Mifflin Company 11-8 The outermost shell in an atom is called the valence shell and the electrons in it are called the valence electrons. These are the electrons involved in forming chemical bonds. All of the elements in a group have the same number of valence electrons and form compounds in a similar fashion. Atomic size is also a periodic property. As you go across a period atomic size decreases. Since the number of positive charges in the nucleus is increasing, the force pulling the electrons in toward the nucleus increases and results in a smaller orbit radius. As you go down the periodic table, atomic size increases since you are adding one shell for each period you add. Figure 11.20 The Relative Sizes of Atoms of Representative Elements Ionization energy (energy required to remove an electron) is also periodic. It increases as you go across a period. More protons in the nucleus hold the electrons with greater attractive force. As you go down the periodic table, ionization energy decreases. As you add shells that are further from the nucleus, the attractive force becomes weaker. Naming Compounds Spotlight Naming Compounds Binary compound formulas are written with the metallic ion first followed by the negative ion. To name a binary compound, write the name of the positive ion and the name of the negative ion. Replace the end of the negative ion name with the suffix ide. Example NaCl - Sodium Chloride Al2O3 - Aluminum Oxide When two nonmetals combine to form a compound, they very often can combine in at least two ratios to form two different compounds. Generally the Greek numeric prefixes are used to distinguish the compounds. Example NO - Nitrogen Monoxide NO2 - Nitrogen Dioxide N2O5 - Dinitrogen Pentoxide An ion is an atom or a group of chemically combined atoms that have an electric charge because they have gained or lost one or more electrons. When more atoms than one combine to form an ion it is called a polyatomic ion. Example Acetate Hydroxide Carbonate Phosphate Ammonium C2H3O2OHCO3-2 PO4-3 NH4+ When writing a compound name with a polyatomic ion, just write the name of the positive ion and then the name of the negative ion. Example Na2CO3 NH4Cl Sodium Carbonate Ammonium Chloride Some compounds have special names that do not follow these rules. Examples are water, ammonia, methane, Sulfuric Acid, and others. For these you must recognize the formula and remember the common name. Study P 306 Key terms P 306 Matching Questions P 307 Multiple Choice Questions P 307 Fill in the Blank Questions P 308 Questions 4, 5, 6, 7, 9, 13, 16, 17, 18, 19, 20, 21, 22, 25, 26, 27, 28, 31, 32 P 309 Exercises 1, 7, 9, 15, 17, 27, 29