Hydrogen Spectrum Lab
advertisement

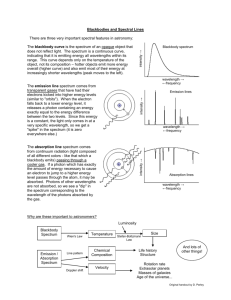
WagenborgHydrogenSpectrumLabPage 1 Name(s)Bill Wagenborg Chemistry 511 The Hydrogen Spectrum Lab Report [Recall that “d” represents the distance between lines in the diffraction grating. If you look at you grating your will read the following listing: “500 lines/mm”, i.e., each millimeter (mm) contains 500 lines. So, the inverse of this, i.e., 1/500 should be the number of millimeters between a line and an adjacent line. Thus, (1/500) mm/line. The result should be: d = 0.002 mm between lines or 2000 nm between lines.] Experimental Data and Calculations: Distance a 91.44 cm 91.44 cm 91.44 cm 91.44 cm 17 cm 23.8 cm 27.6 cm 34 cm Violet Teal Yellow Red 3.66* 10 16 nm 5.05 * 10 16 nm 5.78 * 10 16 nm 6.97 * 10 16 nm 5.43 * 10 19 J 3.94 * 10 19 J 3.44 * 10 19 J 2.85 * 10 19 J Distances b Color Observed Wavelength Energy Violet Wavelength 17 = 3.66* 10 5 cm (1m/100 cm)(10 9 nm/1m)= 3.66 * 10 16 nm .0002cm 2 2 17 9144 . Energy E (6.626 * 10 34 3.0 * 1010 cm / s ) = 5.43 * 10 3.66 * 10 5 cm 19 J Teal 238 . = 5.038 * 10 5 cm(1m/100 cm)(10 9 nm/1m) = 5.038 * 10 16 .0002cm 2 2 238 . 9144 . E (6.626 * 10 34 3.0 * 1010 cm / s ) = 3.94 * 10 5.04 * 10 5 cm 19 nm J 1 WagenborgHydrogenSpectrumLabPage 2 Yellow 27.6 = 5.78 * 10 .0002cm 27.62 9144 . 2 E (6.626 * 10 34 5 cm(1m/100 cm)(10 9 nm/1m) = 5.78 * 10 16 nm 3.0 * 1010 cm / s ) = 3.44 * 10 5.78 * 10 5 cm 19 J Red 34 = 6.97 * 10 5 .0002cm 2 34 2 9144 . cm (1m/100 cm)(10 9 nm/1m)= 6.97 * 10 16 nm 3.0 * 1010 cm / s E (6.626 * 10 34 ) =2.85 * 10 6.97 * 10 5 cm 19 J Show representative wavelength and energy calculations. Then fill in the table with you results. 1. The energy values calculated represent the energy emitted by the electron when it transitions from higher energy level to a lower. The red line in the hydrogen spectrum is the result of a transition from the n=3 to the n=2 energy levels. What energy level transitions are represented by the other lines in the spectrum? Yellow occurs when the level goes from n=4 to n=2. Teal occurs when the level goes from n=5 to n=2. Violet occurs when the level goes from n=6 to n=2. 2. Using your results, explain how ∆E, the difference in energy between levels, varies as n, the energy level, increases. Change in Energy n=5 to n= 4(Violet to Teal) : 1.49 * 10 19 n=4 to n= 3(Teal to Yellow): 5 * 10 20 n=3 to n= 2(Yellow to Red): 5.9 * 10 20 2 WagenborgHydrogenSpectrumLabPage 3 As the energy level increased the difference in energy decreased drastically and then was then very close. The change in energy between n=5 and n= 4 was higher then the change in energy between n=4 and n=3. The change in energy between n=4 and n=3 was slightly lower than the change from n=3 to n=2. (This could be attributed to an error in our original distance measurements.) 3. The spectrum you observed is the visible spectrum for hydrogen, also called the Balmer series. There are two other series for hydrogen: the Paschen and the Lyman series. The Pashcen series occurs at wavelengths longer than those observed in the Balmer series while the Lyman series occurs at wavelengths shorter than those observed in the Balmer series. • In which part of the electromagnetic spectrum is each of these spectral series? Please explain your reasoning. Lyman would be in the gamma rays to ultraviolet part of the electromagnetic spectrum. This is because this part of the spectrum has wavelengths that are shorter than the Balmer Series (visible spectrum). Paschen would occur at the end part of the electromagnetic spectrum (inferred to microwaves and radio waves). This is because the wavelengths in this part are longer than the Balmer Series (visible spectrum). Recall that the lines in Balmer series can be represented as electron energy level changes (transitions) from n-levels > 2 (i.e., 3, 4, 5, 6, …) down to nf = 2. So, for the Balmer series of lines, some of the electron energy level transitions are: ni = 3 ---> nf = 2 ; ni = 4 ---> nf = 2 ; ni = 5 ---> nf = 2 ; etc. One of these “new”spectral series (Paschen or Lyman) involves transitions from higher n-levels to nf =1 and the other involves transitions from higher n-levels to nf = 3. Which electron transitions in the hydrogen atom produce each series? Is this consistent with your answer to #2? Please explain your reasoning. As we go to higher n levels, the change in energy increases by less and less and the wavelengths get smaller and smaller. Thus the series described as the transition from higher levels to n =1 has to be Lyman Series. This is because it was stated that the Lyman series had shorter wavelengths than the Balmer Series. The transition from higher levels to n= 3 must be the Paschen Series since our n level movement is not as high, our change in energy is shorter (not decreasing as much because there is not as many levels)and the wavelengths would be longer. This is because it was stated that the Paschen Series has longer wavelengths than the Balmer Series. This is consistent with my answer to #2. 3 WagenborgHydrogenSpectrumLabPage 4 4