TITLE: Flame Test and Bright Line Spectra Lab
advertisement

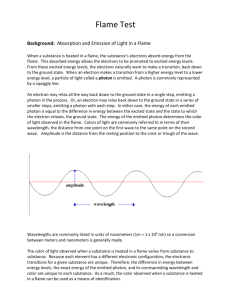
TITLE: Flame Test and Bright Line Spectra Lab Purpose: Please read the lab and write a purpose in your own words. Background Information: Just as a fingerprint is unique to each person, the color of light emitted by metals heated in a flame is unique to each metal. In this laboratory activity, the characteristic color of light emitted for calcium, copper, lithium, potassium, sodium, and strontium will be observed. When a substance is heated in a flame, the substance’s electrons absorb energy from the flame. This absorbed energy allows the electrons to be promoted to excited energy levels. From these excited energy levels, the electrons naturally want to make a transition, or relax, back down to the ground state. When an electron makes a transition from a higher energy level to a lower energy level, a particle of light called a photon is emitted. An electron may relax all the way back down to the ground state in a single step, emitting a photon in the process. Or, an electron may relax back down to the ground state in a series of smaller steps, emitting a photon with each step. In either case, the energy of each emitted photon is equal to the difference in energy between the excited state and the state to which the electron relaxes. The energy of the emitted photon determines the color of light observed in the flame. Because color of light is commonly referred to in terms of their wavelength, equation 1 is used to convert the energy of the emitted photon to its wavelength. E=h or E=hc/ Equation (1) In equation 1, E is the energy of the photon of emitted light H is Plank’s constant (h=6.6262 x 10—34 J sec) NOTE: the value of ‘h’ does not change c is the sped of light (c=3.00 x 108 m/s) is the wavelength of light in meters. is the frequency (1/sec or Hertz) NOTE: Wavelengths are commonly listed in units of nanometers (1m = 1 x 109 nm), so a conversion between meters and nanometers is generally made. The color of light observed when a substance is heated in a flame varies from substance to substance. Because each element has a different electronic arrangement, the electronic transitions for a given metal are unique. Therefore, the exact energy of the emitted photon, and its corresponding wavelength and color are unique to each substance. As a result, the color observed when a substance is heated in a flame can be used as a means of identification. The Electromagnetic Spectrum Visible light is a form of electromagnetic radiation. Other familiar forms of electromagnetic radiation include gamma rays such as those emitted from radioactive materials and from space, X-rays which are used to study bones and teeth, ultraviolet (UV) radiation from the sun, infrared (IR) radiation which is given off in the form of heat, the microwaves used in radar signals and and microwave ovens, and radio waves used for radio and television communication. Together, all forms of electromagnetic radiation make up the electromagnetic spectrum. The visible portion of the electromagnetic spectrum is the only portion that can be detected by the human eye—all other forms of electromagnetic radiation are invisible to the human eye. The visible portion of the electromagnetic spectrum contains all colors of light. The wavelength of the radiation determines the color of the light. In table 1, below are the wavelength ranges for the various colors of light. Table 1 Representative wavelength (nm) 410 470 490 520 565 580 600 650 Wavelength Region, nm Color 400-425 425-480 480-500 500-560 560-580 580-585 585-650 650-700 Violet Blue Blue-green Green Yellow-green Yellow Orange Red PROCEDURE: Part I: 1. Put on you safety glasses. 2. There will be a beaker of SALT solution at each lab station. The six SALTS are NaCl, KCl, LiCl, CaCl2, SrCl2 and CuCl2. In the beaker will be have wood splints in them. There will also be a beaker of water (please place the used splints into the beaker with water when finished). 3. Light the Bunsen burner at the respective station. 4. Place the soaked end of the wood splint into the Bunsen burner flame. Observe the color of the flame. Allow the splint to burn until the color fades. Record the color in you data table. 5. Immerse the wooden splint in the rinse water beaker and place on a paper towel for disposal. 6. Repeat steps 2 to 5 for each station. 7. Obtain a wood splint from the beaker marked unknown. Based upon what you observed in lab, identify the unknown metal contained in this solution. Record your observations and hypothesis in your data table. 8. When you are finished, clean up any burned wood splints and discard them in the trash can. Wash your hands with soap and water. Part II: Complete this procedure at the front lab desk marked “Station 1” 1. At ‘Station 1’ there is a gas discharge rube filled with hydrogen gas. Using the spectroscope on the lab desk, observe the bright line spectrum produced by the hydrogen gas. Identify as many bright lines as you can. On the data table, write each of the individual bright line spectral colors and its corresponding wavelength that you observe. Part III: Complete this procedure at the front lab desk marked “Station 2”. 1. Complete this procedure at the front lab desk marked ‘Station 2’. Observe the bright line spectrum of this unknown gas. Use the classroom spectroscope to observe the bright line spectrum. Identify as many bright line and their corresponding wavelengths as you can and record in data table 3. DATA TABLE I Spectroscope Number: SALT LiCl NaCl KCl CaCl2 SrCl CuCl2 UNKNOWN DATA TABLE II Calibration Measurements Hydrogen Light Source Calibration Color of Line Observed Wavelength Red Cyan Blue Violet Observed Color Actual Wavelength (nm) 656.3 486.1 434.1 410.2 DATA TABLE III UNKNOWN Light Source Calibration Color of Line Observed Wavelength Note: you do not have to fill this entire table; just write what you observe. CALCULATIONS: N/A CONCLUSION: Make sure you include this. QUESTIONS: 1. Briefly explain why, when an element is heated, it gives off line spectra of certain wavelengths instead of a continuous spectrum. (HINT: read section 4-3 in your book about the Bohr Model of the atom). 2. How are bright line spectra useful? (HINT: Astronomy) 3. What are the four characteristics of a wave? 4. What is the speed of light in meters per second? 5. Define quantum. 6. Define ground state. 7. Define excited state. 8. The lines from an atomic line spectrum, where do these lines come from? Explain in terms of electron movement. The purpose of this lab is (1) to measure the wavelengths of the most intense bright line spectra given off by some elements on the periodic table and (2) to determine the identity of an unknown element by measuring the wavelengths of bright line spectra given off by this element and comparing it to known bright line spectra.)