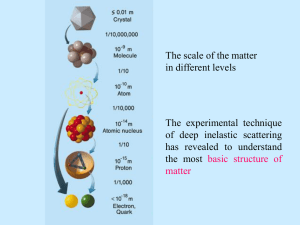
1. Two particles are observed to emerge from a nuclear interaction
advertisement
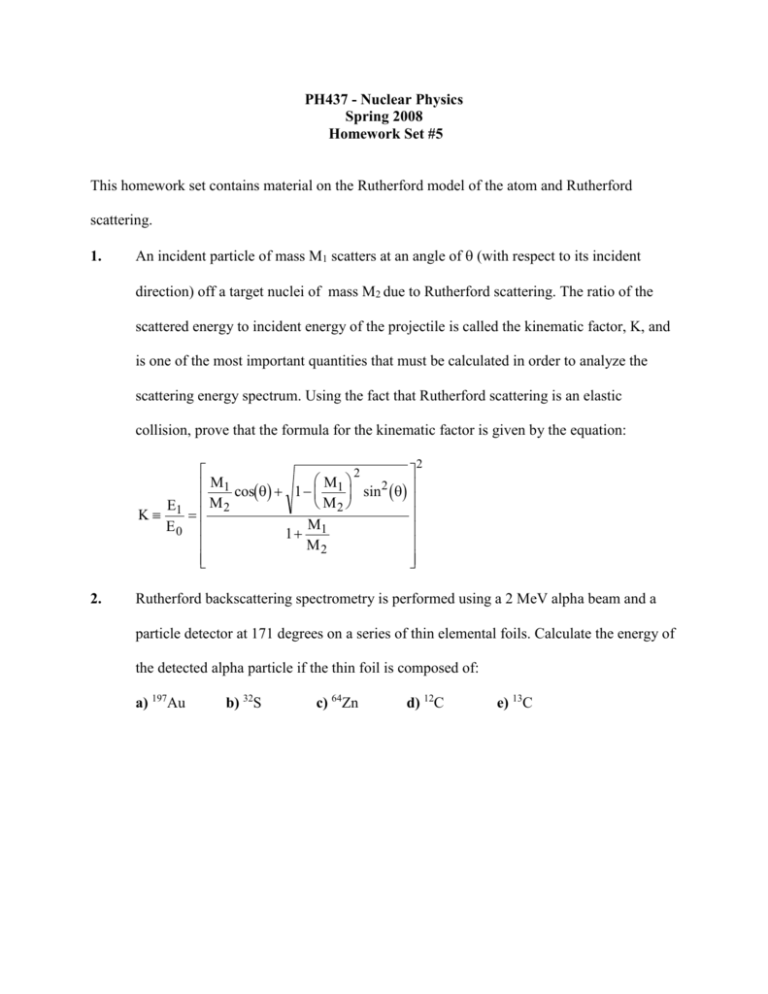
PH437 - Nuclear Physics Spring 2008 Homework Set #5 This homework set contains material on the Rutherford model of the atom and Rutherford scattering. 1. An incident particle of mass M1 scatters at an angle of (with respect to its incident direction) off a target nuclei of mass M2 due to Rutherford scattering. The ratio of the scattered energy to incident energy of the projectile is called the kinematic factor, K, and is one of the most important quantities that must be calculated in order to analyze the scattering energy spectrum. Using the fact that Rutherford scattering is an elastic collision, prove that the formula for the kinematic factor is given by the equation: K 2. E1 E0 2 M1 cos 1 M1 sin 2 M2 M2 M1 1 M2 2 Rutherford backscattering spectrometry is performed using a 2 MeV alpha beam and a particle detector at 171 degrees on a series of thin elemental foils. Calculate the energy of the detected alpha particle if the thin foil is composed of: a) 197Au b) 32S c) 64Zn d) 12C e) 13C 3. The following research breakthrough was reported in “Physics News Update” by the American Institute of Physics on January 8, 1997: A NEW ELECTROLUMINESCENT DEVICE: A new electroluminescent device uses 1/10th the voltage of previous devices. Head-mounted displays (small enough to fit into a visor) in automobile, aircraft, and microsurgery environments won’t be practical until the conversion of electricity into tiny parcels of light can be done using small currents and voltages. At the heart of a thin-film electroluminescent (TFEL) device is a host material such as ZnS doped with luminescing centers such as Mn atoms. On either side of this material are insulating layers which serve as suppliers of electrons. High electric fields, supplied by a voltage applied across the whole sandwich, launch electrons into ZnS where they strike a manganese atom, which emits a photon. A new TFEL concept developed at Georgia Tech (Christopher Summers, chris.summers@gtri.gatech.edu) employs much thinner insulating layers, which permits the electrons to reach their necessary velocity using much less voltage: 1525 V instead of the customary 150-200 V. The efficiency of the new device is still low and the cost of growing the crystalline insulating layers is comparatively high, but the low-voltage requirements, and the smaller circuitry this will permit, may make the approach worthwhile. (C.J. Summers et al., upcoming article in Applied Physics Letters). After reading this report, Dr. Donald Duck, Director of Materials Research at Acme Corp., contacted Dr. Dave Morton of the Army Research Lab and received funding to develop quality manganese doped ZnS films for ARL. Dr. Duck has made thin films of ZnS on a thin carbon foil backing. Dr. Duck needs to know the stoichiometry of his film (ratio of S to Zn), the manganese doping concentration (ratio of Mn to S) and is also worried about possible processing contamination by the following impurities: Au, O, H, and Al. To answer these questions, Dr. Duck has supplied your boss, Dr. Wiley Coyote, with a thin test film for analysis using Rutherford backscattering. Mr. T. Devil, your RBS technician, has obtained an RBS spectrum with peak energies and heights listed in the following table using a 2 MeV alpha beam with the detector at 171 degrees. Dr. Coyote has asked you to analyze the spectrum and supply Dr. Duck with the following information: a) the stochiometry of the film (ratio of S to Zn) b) the doping concentration of the Mn (ratio of Mn to Zn) c) the name of any impurities detected in the film d) tell Dr. Duck if there are any impurities in his list that you can not detect with RBS. Peak Energy(MeV) Counts Peak Energy (MeV) Counts 0.504 894 1.213 18620 0.565 10 1.249 848 0.724 40 1.496 1453 1.100 1240 1.568 71240 4. The following experimental data is similar to that obtained using the LC-400 Van de Graaff accelerator by CDT Bull during the spring of 1996 for protons scattered off a carbon foil as a function of proton beam energy. Beam Energy (keV) Counts Beam Energy (keV) Counts 255 237601 355 115096 265 213599 365 107074 275 198347 375 103147 285 184488 385 87232 295 168572 400 79313 305 162859 410 43635 315 152683 420 31718 325 142864 430 67415 335 128982 440 144886 345 122999 450 207407 Assuming that all data points were taken with the same experimental geometry and for the same number of incident protons, determine all energy regions where the scattering data indicates that the scattering between the proton and the carbon atom was Rutherford. (Hint: Plot the data on the appropriate type of graph and check for the Rutherford scattering energy dependence) 5. Determine the Rutherford scattering cross sections for 150 keV protons scattered at 171 degrees off 197Au and 12C. Remember to apply electron shielding corrections if necessary.