Chemistry Math Review Study Guide with Key
advertisement
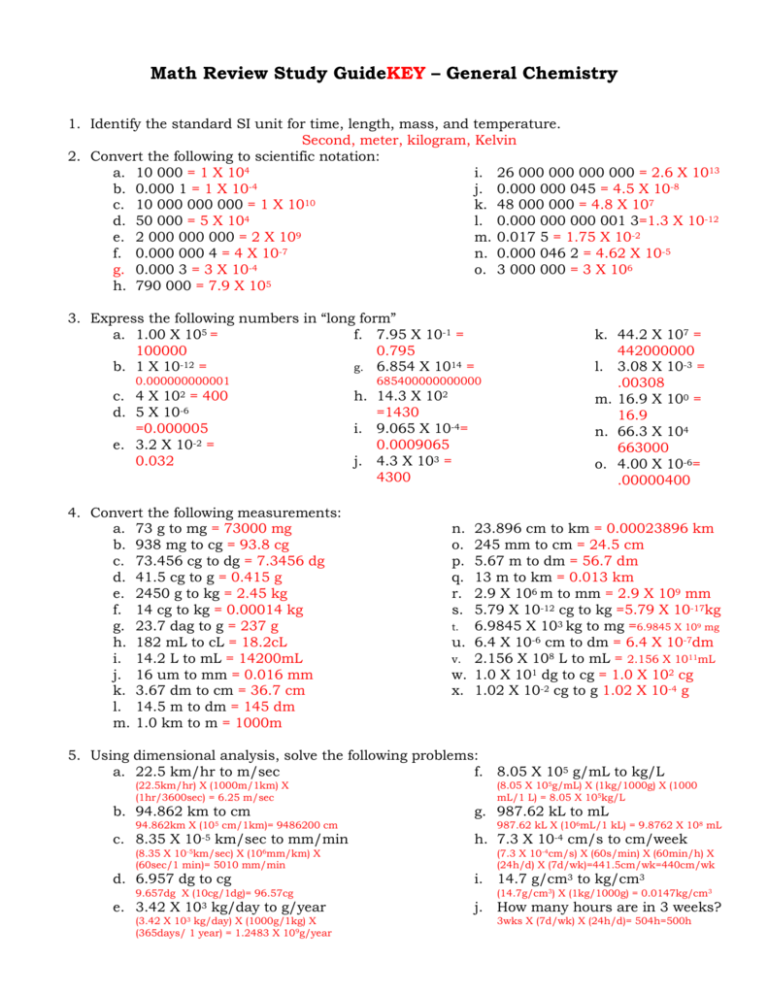
Math Review Study GuideKEY – General Chemistry 1. Identify the standard SI unit for time, length, mass, and temperature. Second, meter, kilogram, Kelvin 2. Convert the following to scientific notation: a. 10 000 = 1 X 104 i. 26 000 000 000 000 = 2.6 X 1013 -4 b. 0.000 1 = 1 X 10 j. 0.000 000 045 = 4.5 X 10-8 c. 10 000 000 000 = 1 X 1010 k. 48 000 000 = 4.8 X 107 d. 50 000 = 5 X 104 l. 0.000 000 000 001 3=1.3 X 10-12 9 e. 2 000 000 000 = 2 X 10 m. 0.017 5 = 1.75 X 10-2 -7 f. 0.000 000 4 = 4 X 10 n. 0.000 046 2 = 4.62 X 10-5 g. 0.000 3 = 3 X 10-4 o. 3 000 000 = 3 X 106 h. 790 000 = 7.9 X 105 3. Express the following numbers in “long form” a. 1.00 X 105 = f. 7.95 X 10-1 = 100000 0.795 -12 b. 1 X 10 = g. 6.854 X 1014 = 0.000000000001 c. 4 X 102 = 400 d. 5 X 10-6 =0.000005 e. 3.2 X 10-2 = 0.032 4. Convert the following measurements: a. 73 g to mg = 73000 mg b. 938 mg to cg = 93.8 cg c. 73.456 cg to dg = 7.3456 dg d. 41.5 cg to g = 0.415 g e. 2450 g to kg = 2.45 kg f. 14 cg to kg = 0.00014 kg g. 23.7 dag to g = 237 g h. 182 mL to cL = 18.2cL i. 14.2 L to mL = 14200mL j. 16 um to mm = 0.016 mm k. 3.67 dm to cm = 36.7 cm l. 14.5 m to dm = 145 dm m. 1.0 km to m = 1000m 685400000000000 h. 14.3 X 102 =1430 i. 9.065 X 10-4= 0.0009065 j. 4.3 X 103 = 4300 n. o. p. q. r. s. t. u. v. w. x. k. 44.2 X 107 = 442000000 l. 3.08 X 10-3 = .00308 m. 16.9 X 100 = 16.9 n. 66.3 X 104 663000 o. 4.00 X 10-6= .00000400 23.896 cm to km = 0.00023896 km 245 mm to cm = 24.5 cm 5.67 m to dm = 56.7 dm 13 m to km = 0.013 km 2.9 X 106 m to mm = 2.9 X 109 mm 5.79 X 10-12 cg to kg =5.79 X 10-17kg 6.9845 X 103 kg to mg =6.9845 X 109 mg 6.4 X 10-6 cm to dm = 6.4 X 10-7dm 2.156 X 108 L to mL = 2.156 X 1011mL 1.0 X 101 dg to cg = 1.0 X 102 cg 1.02 X 10-2 cg to g 1.02 X 10-4 g 5. Using dimensional analysis, solve the following problems: a. 22.5 km/hr to m/sec f. 8.05 X 105 g/mL to kg/L (22.5km/hr) X (1000m/1km) X (1hr/3600sec) = 6.25 m/sec b. 94.862 km to cm 94.862km X (105 cm/1km)= 9486200 cm c. 8.35 X 10-5 km/sec to mm/min (8.35 X 10-5km/sec) X (106mm/km) X (60sec/1 min)= 5010 mm/min d. 6.957 dg to cg 9.657dg X (10cg/1dg)= 96.57cg e. 3.42 X 103 kg/day to g/year (3.42 X 103 kg/day) X (1000g/1kg) X (365days/ 1 year) = 1.2483 X 109g/year (8.05 X 105g/mL) X (1kg/1000g) X (1000 mL/1 L) = 8.05 X 105kg/L g. 987.62 kL to mL 987.62 kL X (106mL/1 kL) = 9.8762 X 108 mL h. 7.3 X 10-4 cm/s to cm/week i. j. (7.3 X 10-4cm/s) X (60s/min) X (60min/h) X (24h/d) X (7d/wk)=441.5cm/wk=440cm/wk 14.7 g/cm3 to kg/cm3 (14.7g/cm3) X (1kg/1000g) = 0.0147kg/cm3 How many hours are in 3 weeks? 3wks X (7d/wk) X (24h/d)= 504h=500h 6. Calculate the percent error in the following problems: a. What is the % error for a mass measurement of 15.6 g, given that the correct value is 26.9g? /15.6-26.9/ ÷ 26.9 X 100 = 42.0% b. A volume is measured experimentally as 4.26 mL. What is the % error, given that the correct value is 4.15mL? /4.26-4.15/ ÷ 4.15 X 100 = 2.65% c. A student measures the mass and volume of a substance and calculates its density as 1.40 g/mL. The correct value of the density is 1.36 g/mL. What is the percent error of the student’s measurement? /1.40-1.36/ ÷ 1.36 X 100 = 2.94% d. A student measures the mass of a sample as 9.67 g. Calculate the percent error, given that the correct mass is 9.82 g. /9.67-9.82/ ÷ 9.82 X 100 = 1.53% e. What is the % error of a length measurement of 0.229 cm if the correct value is 0.225 cm? /0.229-0.225/ ÷ 0.225 X 100 = 1.78% 7. Determine the number of significant figures in each of the following numbers: a. 5.432 g 4 i. 144 kg b. 40.319 g 5 j. 2500 cm 3 c. 146 cm 3 k. 2500.0 cm d. 0.189 kg 3 l. 1.04 X 10 14 g e. 429.3 g 4 m. 3.58 X 10-9 nm f. 2873.0 cm3 5 n. 48.571 93 kg 3 g. 99.9 cm 3 o. 8365.6 g h. 0.000 235 g 3 p. 0.002 300 mg 3 2 5 3 3 7 5 4 8. Round each figure to three significant figures: a. 0.003 210 g = 0.00321 g b. 3.875 4 kg = 3.88 kg c. 219 034 m =219000 m d. 25.38 L = 25.4 L e. 0.087 63 cm = 0.0876 cm f. 0.003 109 mg = 0.00311 mg 9. Round each number to four significant figures: a. 431 801 kg = 431800 kg b. 10 235.0 mg = 10240 mg c. 1.0348 m = 1.025 m d. 0.004 387 010 cm = 0.004387cm e. 0.000 781 00 mL=0.0007810mL f. 0.009 864 1 cg = 0.009864 cg 10. Round each of the answers in the following problems to the correct number of significant figures: a. 7.31 X 104 + 3.23 X 103 89.5 cm3 = 7.63 X 104 e. 3.40 mg + 7.34 mg + 6.45 mg b. 8.54 X 10-3 – 3.41 X 10-4 17.19 mg -3 = 8.20 X 10 f. 45 m X 72 m X 132 m c. 4.35 dm X 2.34 dm X 7.35 dm 430000 m3 3 = 74.8 dm g. 38736 km / 4784 km d. 4.78 cm X 3.218 cm X 5.82 cm 8.097 11. Write a. b. c. the conversion factor that converts: Grams to kilograms 1kg/1000g Kilograms to grams 1000g/1kg Millimeters to meters 1m/1000mm d. Meters to millimeters 1000mm/1m e. Centiliters to liters 1L/100cL f. Liters to centiliters 100cL/1L 12. Calculate the density of the following: a. A sample of aluminum metal has a mass of 8.4 g. The volume of the sample is 3.1 cm3. Calculate the density of aluminum. D = m/V = 8.4g/3.1cm3 = 2.7g/cm3 b. 8.0 cm3 of platinum are found to have a mass of 171.2 g. Determine the density. D = 171.2 g/8.0 cm3 = 21 g/cm3 c. 50.0 cm3 of lead have a mass of 565 g. What is the density of the lead? D = 565 g/50.0 cm3 = 11.3 g/cm3 d. Diamond has a density of 3.26 g/cm3. What is the mass of a diamond that has a volume of 0.350 cm3? m = DV = 3.26 g/cm3 X 0.350 cm3 = 1.14 g e. What is the volume of a sample of liquid mercury that has a mass of 76.2 g, given that the density of mercury is 13.6 g/mL? V = m/D = 76.2 g/13.6 g/mL = 5.60 mL







