spectrum atoms
advertisement

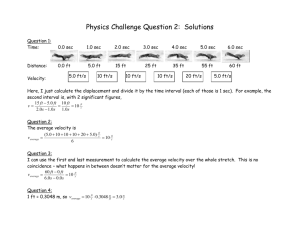
Chapter 12 Nuclear Magnetic Resonance Spectroscopy Multiple Choice 1. How many sets of equivalent hydrogen atoms are there for 2-propanol? (Sec. 12.6) a) b) c) d) 2 3 4 8 2. How many sets of equivalent hydrogen atoms are there for the following compound? (Sec. 12.6) CH3 a) b) c) d) 3 4 5 6 3. How many sets of signals are there in the 1H-NMR spectrum for 1,1-dichlorocyclohexane? (Sec. 12.6) a) b) c) d) 2 3 4 6 4. How many sets of signals are there in the 1H-NMR spectrum for diisopropyl ether? (Sec. 12.6) a) b) c) d) 1 2 3 6 5. How many sets of equivalent carbon atoms are there for the following compound? (Sec. 12.10) CH3 OH a) b) c) d) 3 4 5 6 6. How many sets of equivalent carbon atoms are there for 1-bromo-3-chlorocyclohexane? (Sec. 12.10) a) b) c) d) 3 4 5 6 138 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 7. A compound gave two sets of signals in the 1H-NMR spectrum with an integration ratio of 60 to 10. Which is the most likely structure for the compound? (Sec. 12.6, 12.7) O a) CH3CH2CH2CCH2CH2CH3 b) H3C OH CH3 O H3C CHCCH d) c) O CH3 H3C CH3 CH3 8. Which are the relative areas of integration in the 1H-NMR spectrum for 2-chloro-3-methylbutane? (Sec. 12.7) a) b) c) d) 9:1:1 3:3:3:1:1 6:3:2 6:3:1:1 9. Which is the order of increasing values downfield from TMS for the methyl groups shown (lowest first)? (Sec. 12.8) CH3 Br CH3 I CH3 OH CH3 F I a) b) c) d) II III IV I, III, IV, II II, I, III, IV IV, II, III, I III, IV, II, I 10. Which is the order in increasing chemical shift values in the 1H-NMR spectrum for the indicated hydrogen atoms (lowest first)? (Sec. 12.8) O CH3 CH CH CH2 COH I a) b) c) d) II III IV IV, II, III, I I, III, II, IV III, I, IV, II II, IV, I, III 139 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 11. Which is the splitting pattern for the indicated hydrogen atom? (Sec. 12.9) O CH3 CH3COCH CH3 a) b) c) d) septet quartet doublet singlet 12. Which is the splitting pattern for the hydrogen atoms in 2,2-dibromopropane? (Sec. 12.9) a) b) c) d) septet quartet doublet singlet 13. The splitting pattern for a compound with molecular formula C3H5OCl gave a quartet and a triplet. Which is the most likely structure for this compound? (Sec. 12.9) O a) CH3CCH2Cl b) O Cl O O c) CH3CH2CCl d) ClCH2CH2CH 14. Which compounds show signal splitting in the 1H-NMR spectrum? (Sec. 12.9) CH3 O O CH3 CH3COC CH3 CH3 CH3 CH3 C COCH2CH3 CH3O C CH3 CH3 I CH3 III II a) b) c) d) OCH3 IV I, II III, IV I, III II, IV 15. Which compound has a singlet in the 1H-NMR at approximately 2.0 ppm and integrates to 3 hydrogens? (Sec. 12.8, 12.9) O O a) CH3CHOCH CH3 b) CH3COCH2CH3 O c) CH3CH2CH2OCH O d) CH3OCCH2CH3 140 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 16. Which compound has four sets of signals in the 1H-NMR spectrum? (Sec. 12.6) O O a) CH3CHOCH CH3 b) CH3COCH2CH3 O c) CH3CH2CH2OCH O d) CH3OCCH2CH3 17. Which compounds have 3 signals in the 13C NMR spectrum? (Sec. 12.10) HO OH OH OH OH OH OH I II a) b) c) d) IV III I, II I, II, III I, III I, II, III, IV 18. Which is the order of increasing values in the 13C NMR spectra for the following carbon atoms? (Sec. 12.8) CH4 I a) b) c) d) CH3F II CH3Br III CH3OH IV CH3Cl V III, V, II, IV, I I, IV, II, V, III V, IV, II, III, I I, III, V, IV, II 19. A compound has a single signal in the 13C NMR spectrum and a single signal in the 1H-NMR spectrum. Which is most likely the compound? (Sec. 12.6, 12.10) a) b) c) d) dimethyl ether diethyl ether 2,2-dimethylpropane methyl ethanoate 20. A compound has two signals in the 13C NMR spectrum and a single signal in the 1H-NMR spectrum. Which is most likely the compound? (Sec. 12.6, 12.10) a) b) c) d) dimethyl ether diethyl ether 2,2-dimethylpropane methyl ethanoate 141 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 21. A compound has three signals in the 13C NMR spectrum and two signals in the 1H-NMR spectrum. Which is most likely the compound? (Sec. 12.6, 12.10) a) bromobenzene b) para dibromobenzene c) ortho dibromobenzene d) meta dibromobenzene 22. Which are the characteristic shift ranges for the indicated hydrogen atoms? (Sec. 12.8) O H3C CCH2CH2CH3 H I III IV II a) b) c) d) I = 0.8-1.0, II = 6.5-8.5, III = 2.2-2.5, IV = 2.1-2.3 I = 0.8-1.0, II = 2.2-2.5, III = 6.5-8.5, IV = 2.1-2.3 I = 2.2-2.5, II = 6.5-8.5, III = 0.8-1.0, IV = 2.1-2.3 I = 2.2-2.5, II = 6.5-8.5, III = 2.1-2.3, IV = 0.8-1.0 23. The compounds shown below can be distinguished by which 1H-NMR characteristics? (Sec. 12.11) O O I) number of signals II) splitting III) integration IV) chemical shift a) b) c) d) I, II, IV I, IV II IV 142 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 24. The compounds shown below can be distinguished by which 1H-NMR characteristics? (Sec. 12.11) Cl Cl Cl Cl I) number of signals II) splitting III) integration IV) chemical shift a) b) c) d) I, II I, IV I, II IV 25. The compounds shown below can be distinguished by which 1H-NMR characteristics? (Sec. 12.11) O O O I) number of signals II) splitting III) integration IV) chemical shift a) b) c) d) I, II, III I, III, IV I, II IV Fill in the Blank 1. The number of 1H signals for the following molecule is _____________. (Sec. 12.6) O O 143 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 2. The number of 1H signals for the following molecule is _____________. (Sec. 12.6) CH3 CH3 3. The number of 1H signals for isopropyl ethyl ether is _____________. (Sec. 12.6) 4. The splitting of the three 1H signals of methyl propanoate are (left to right as drawn) _____, ______, ______. (Sec. 12.9) O O 5. The splitting of the three 1H signals of tetrahydropyran are (top to bottom as drawn) _____, ______, ______. (Sec. 12.9) O 6. The integration of the 1H signal at 2.2 ppm for 3-pentanone is _________. (Sec. 12.7) O 7. The integration of the 1H signal at 3.8 ppm for dipropyl ether is _________. (Sec. 12.7, 12.8) 8. The number of 13C signals for meta dimethoxybenzene is ________. (Sec. 12.10) 9. The expected chemical shift range (in ppm) for the indicated carbon of ethyl benzoate is ___________. (Sec. 12.10) O O 144 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 10. The expected chemical shift range (in ppm) for the indicated protons of E-2-pentene is ___________. (Sec. 12.8) H H True-False 1. The number of 1H-NMR signals for propyl propanoate is 3. (Sec. 12.6) O O 2. The number of 1H-NMR signals for para methoxytoluene is 4. (Sec. 12.6) 3. The splitting of the signal for the indicated protons of 3-methylcyclohexanone is a quartet. (Sec. 12.9) O H CH3 4. The splitting of the signal for the indicated protons of isopropyl formate is a septet. (Sec. 12.9) CH3 O H O H CH3 5. The integration of the signal that includes the indicated protons of isopropyl formate is 3. (Sec. 12.7) CH3 O H O H CH3 145 Chapter 12 Nuclear Magnetic Resonance Spectroscopy 6. The integration of the signal that includes the indicated protons of meta hydroxybenzene is 2. (Sec. 12.7) OH OH H 7. Ethyl 3-oxopentanoate has 5 proton signals and 5 carbon signals in the NMR spectra. (Sec. 12.6, 12.10) O O O 8. 1,4-Cyclohexanedione has 2 proton signals and 4 carbon signals in the NMR spectra. (Sec. 12.6, 12.10) O O 9. A 1H-NMR shows a single signal and a 13C-NMR shows two signals. A possible structure for this compound is, O (Sec. 12.6, 12.10) 10. A 1H-NMR shows a single signal and a 13C-NMR shows two signals. A possible structure for this compound is, O (Sec. 12.6, 12.10) 146 Chapter 12 Nuclear Magnetic Resonance Spectroscopy Answers Multiple Choice 1. b 2. d 3. b 4. b 5. c 6. d 7. d 8. d 9. b 10. b 11. a 12. d 13. c 14. d 15. b 16. c 17. d 18. d 19. a 20. c 21. c 22. d 23. d 24. b 25. b Fill in the Blank 1. 4 2. 4 3. 4 4. t, q, s 5. t, q(5), q(5) 6. 4 7. 4 8. 5 9. 165-200 ppm 10. 5-5.7 ppm True-False 1. F 2. T 3. F 4. T 5. F 6. T 7. F 8. F 9. T 10. T 147