2. Experimental
advertisement

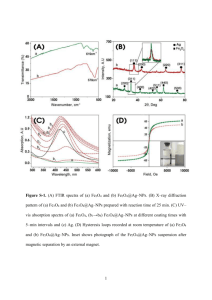
PHYSICS, CHEMISTRY AND APPLICATION OF NANOSTRUCTURES, 2011 INFLUENCE OF SYNTHESIS CONDITIONS ON THE SIZE, MORPHOLOGY AND STRUCTURE OF IRON OXIDE PARTICLES A. FILIPOVICH, M. IVANOVSKAYA, D. KOTSIKAU, V. PANKOV Research Institute for Physical Chemical Problems, Belarusian State University 14 Leningradskaya, Minsk, 220030, Belarus. E-mail: ivanovskaya@bsu.by The possibility to control the structure, size and morphology of iron oxide nanoparticles prepared by the sol-gel approach has been studied. Two procedures were used to obtain γFe2O3 and Fe3O4 samples: i) spray pyrolysis of FeII + FeIII salt solutions or Fe3O4·nH2O colloidal solutions; ii) chemical precipitation of FeII or FeII + FeIII hydroxides with subsequent peptization of the sediment and drying. SiO2 sol, chloride ions and surfaceactive agents (surfactants) were added into starting solutions to modify the surface of the resulting iron oxide nanoparticles and to control their size. Structural features of the samples were examined by XRD, TEM and SEM, IR spectroscopy and Mössbauer spectroscopy. 1. Introduction Nanoparticles of γ-Fe2O3 are among the most frequently studied systems due to their considerable value for biomedical applications [1−4]. In this regard, there are certain requirements for particle size, surface and magnetic parameters of γ-Fe2O3. The magnetic characteristics can be managed within some limits by changing the size, shape and structure of γ-Fe2O3 nanoparticles. The aim of the work is a comparative evaluation of the size, morphology and structure of γ-Fe2O3 particles obtained by different variants of the sol-gel synthesis. 2. Experimental The γ-Fe2O3 powders were obtained by: i) combined precipitation of FeII/FeIII hydroxides with ammonia solution and transformation of the precipitate into a sol form by the peptization with HNO3 and ultrasonic treatment; ii) spray pyrolysis of an aerosol of aqueous Fe(NO3)3 + FeSO4 solution; iii) spray pyrolysis of the Fe3O4∙nH2O sol, obtained by the method i. The effect of additives, such as silica sol and chloride ions, on the formation of γ-Fe2O3 particles was studied. The samples were characterized by means of X-ray diffraction (XRD) analysis, Fourier-transform infrared spectroscopy (FTIR), transmission and scanning electron microscopy (TEM and SEM), and Mössbauer spectroscopy. XRD analysis was carried out on a HZG−4A diffractometer by using Ni-filtered Co K radiation. IR-spectra were recorded on an AVATAR FTIR-330 1 2 spectrometer. TEM/ED examinations were performed with a LEO 906E and a JEOL 4000 EX transmission electron microscopes. The resonance spectra were recorded in air at 298 K and processed by using a SM2201 Mössbauer spectrometer equipped with a 15 mCi 57Co (Rh) source. 3. Results and Discussion 3.1. Sol-gel synthesis of γ-Fe2O3 EM data evidence that the sol, prepared by chemical precipitation of FeII or FeII + FeIII hydroxides with subsequent peptization of the sediment, contains spherically shaped particles of Fe3O4 with a size of 6−7 nm (Figure 1). After annealing at 300°C, Fe3O4 completely transforms into γ-Fe2O3. The formation of γ-Fe2O3 and the absence of Fe2+ in the sample were confirmed by Mössbauer spectroscopy (Table 1), which indicates the presence of a cubic phase of iron oxide (δ = 0.34 mm/s, Δ = −0.03 mm/s, B = 49.1 T). The γ-Fe2O3 → α-Fe2O3 transition starts after annealing at 400°C. Table 1. Phase composition and particle size of γ-Fe2O3 as a function of annealing temperature according to XRD data 50°C 300°C Phase d, nm Fe3O4 6-7 Phase γ-Fe2O3 400°C 500°C 800°C d, nm Phase d, nm Phase d, nm Phase d, nm 6-7 γ-Fe2O3 α-Fe2O3 7-8 − α-Fe2O3 γ-Fe2O3 30-40 10 α-Fe2O3 70-80 The growth of iron oxide particles and the transformation of γ-phase into αphase takes place when the annealing temperature is increased. Figure 1. TEM image of the γ-Fe2O3 sample, annealed at 300°C. Figure 2. SEM image of the products obtained by aerosol pyrolysis of Fe(NO3)3 + FeSO4 solution with the addition of SiO2. 3 3.2. Spray pyrolysis of solutions of FeII and FeIII salts The products of the pyrolysis of FeII and FeIII salts solution at 350−400°C are Xray amorphous phase and represent spherically shaped particles with the predominant size of 0.5−1.0 mm (Figure 2). The adition of silica sol to the salts solution does not prevent the growth of the particles. The IR-spectroscopy data indicate the presence of γ-Fe2O3 (absorption bands at 480 and 660 cm−1), α-Fe2O3 (530 cm-1) and sulfate ions (595 cm-1) in the pyrolysis products. 3.3. Spray pyrolysis of Fe3O4∙nH2O sol According to the XRD data, the products of the pyrolysis of Fe3O4∙nH2O sol carried out at 350°C appeared to be crystalline and contained the particles of ferrimagnetic γ-Fe2O3 phase. The primary particle size, according to TEM, is 7−8 nm (Figure 3). These particles are combined to form spherical globules. The IR spectroscopy confirms the formation of γ-Fe2O3 phase structure (560−580 cm−1, 635 cm−1, 690 cm−1) and the absence of α-Fe2O3 phase in the pyrolysis products at 350°C. Silica sol addition to the initial Fe3O4 sol does not prevent the aggregation of γ-Fe2O3 particles. Only the addition of the chloride ions (as KCl) to the Fe3O4∙nH2O + SiO2∙nH2O sol mixture inhibits the aggregation of iron oxide particles. According to the TEM data, synthesis of γ-Fe2O3 by pyrolysis of Fe3O4∙nH2O sol in the presence of chloride ions results in particles of 20−80 nm with the predominant size of 30−45 nm (Figure 4). Particles of this size are preferred for biomedical applications [4, 5]. Figure 3. TEM image of the products obtained by spray pyrolysis of Fe3O4∙nН2О sol. Figure 4. TEM image of the products obtained by spray pyrolysis of Fe3O4∙nН2О sol with the addition of SiO2 and KCl. 4 4. Conclusion 1. Sol-gel method, which includes the combined hydrolysis of Fe II and FeIII salts with the formation of Fe3O4, transformation of the sediment in colloidal state by peptization, and heating at 300°C, leads to the formation of γ-Fe2O3 particles with d = 6−7 nm. 2. Pyrolysis of the Fe(NO3)3 + FeSO4 solution does not lead to a singlephase product; thus, along with the ferrimagnetic γ-Fe2O3 phase it contains αFe2O3 and FeSO4. 3. Spray pyrolysis of Fe3O4∙H2O sol leads to the formation of aggregated spherical particles of γ-Fe2O3 with d = 0.5−1.0 mkm. Primary particle size is 7−8 nm. The γ-Fe2O3−SiO2 nanocomposites were formed by adding silica sol to the initial Fe3O4 sol. The silica sol does not prevent γ-Fe2O3 particles from aggregation. 4. Chloride ions, introduced into the Fe3O4 sol, prevent effectively the aggregation of γ-Fe2O3 particles, formed by spray pyrolysis. References 1. 2. 3. 4. P.C. Morais, Bull. Pol. Ac.: Tech. 56 (3), 253 (2008). J. Yang, J. Gunn, S.R. Dave et al., Analyst 133, 154 (2008). D. Bahadur et al, Pramana − J. Phys. 65 (4), 663 (2005). N. Sounderya, Y. Zhang, Recent Patents on Biomedical Engineering, 1, 34 (2008). 5. T.-J. Yoon, J. S. Kim, B. G. Kim et al, Angew. Chem. Int. Ed. 44, 1068 (2005).