Phyto-PAM Lab
advertisement

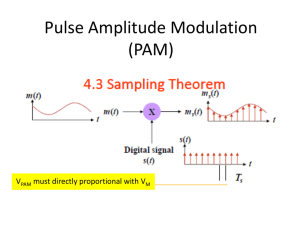
Photosynthetic Efficiency (FV/FM) as an indicator of physiological status Background: One parameter that can be used to assess the physiological status of phytoplankton is the photosynthetic efficiency of photosystem II, also known as FV/FM. One of three things will happen to light energy absorbed by chlorophyll a: it will be used for photochemistry, given off as fluorescence, or given off as heat. If we assume that energy given off as heat is negligible, then fluorescence can be used to look at the photochemistry of the cell. Light will excite chlorophyll a into its excited state where it can then transfer an electron into the electron transport chain (ETC) for the production of ATP, the reduction of NADP and the ultimate production of glucose. As the electron is transferred through the ETC, the initial electron acceptor becomes open to accept a new electron. Under high light this system can become saturated because all reaction centers are closed (occupied by electrons). When the reaction centers are closed, new electrons cannot be accepted at the rate the chlorophyll is being excited. When this happens, the chlorophyll will release the excess energy in the form of light. This light is the fluorescence that we measure using the fluorometer or the flow cytometer that you have used in previous labs. The diagram below is a summary of the light reactions. High-energy components of the system are shown near the top of the diagram. 1 The efficiency with which light energy is utilized is a function of how “healthy” the cell is. When cells are under stress, the system becomes saturated more easily and the system does not process light as efficiently. To measure photosynthetic efficiency, a sample is kept in the dark for a period of time so that all of electron acceptors in the reaction centers are “open” and able to accept electrons. The fluorescence is measured at this point when the most light energy can be accepted into the ETC for photochemistry. The sample is then given a pulse of light until all of the electron acceptors are saturated and the reaction centers are closed. All absorbed light energy at this point will then be given off as fluorescence. The difference between the initial fluorescence (F0) and the maximum fluorescence (FM) is known as the variable fluorescence (FV = FM – F0). FV normalized to FM (FV/FM) is a measure of the photosynthetic efficiency of photosystem II. Changes in FV/FM will occur relatively rapidly when cells are under stress and so this parameter is often one of the earliest and most sensitive indicators of physiological stress. 1 Image taken from: http://faculty.clintoncc.suny.edu/faculty/Michael.Gregory/files/Bio%20101/Bio%20101%20Lectures/Photosynthesis/photosyn.ht m This is a nice site for a review of photosynthesis. 1. Pulse-Amplitude-Modulation (PAM) fluorometry Today’s Lab: Today you will treat a culture of Phaeodactylum tricornutum (a diatom) with a range of temperatures (causing heat-stress or heat shock) and concentrations of hydrogen peroxide (free radicals causing oxidative stress). You will use a PAM fluorometer to evaluate how quickly the cells become stressed under each of the treatments and determine if there are any synergistic effects of heat stress and oxidative stress. The PAM fluorometer will measure F0 and then give the sample a saturating pulse of light to measure FM. It will calculate FV/FM for you. For your first and last timepoint, you will also determine FV/FM with an alternative method using DCMU (see below). Treatments: You will have 3 temperature treatments: control (20ºC); 35ºC; 40ºC and 4 H2O2 treatments: control (0M); 184M; 230M; 276M final concentration. This will give us a total of 12 treatments: 1. 2. 3. 4. 20ºC & 0M 20ºC & 184M 20ºC & 230M 20ºC & 276M 5. 6. 7. 8. 35ºC & 0M 35ºC & 184M 35ºC & 230M 35ºC & 276M 9. 40ºC & 0M 10. 40ºC & 184M 11. 40ºC & 230M 12. 40ºC & 276M Each student will be assigned one treatment and will perform a time-course experiment. We will then pool the data from all students for a complete dataset to examine. Procedure: 1. From the same batch of culture each person will measure out 70 mL of sample into a flask – label the flask with your treatment number 2. Add H2O2 to reach your assigned final concentration 184M = 10L 1.288M H2O2 to 70mL sample 230 M = 12.5L 1.288M H2O2 to 70mL sample 276M = 15L 1.288M H2O2 to 70mL sample 3. Divide your sample into 10 test-tubes with 4mL each (save the flask with the remaining sample) 4. Make an additional 2 test-tubes with 6mL each 5. Label your tubes and place them in the water bath set at your assigned temperature 6. Take a time-0 (T0) measurement of FV/FM with the PAM fluorometer (record the time) 7. Take another reading every 15-20 minutes, recording the time 8. Record your measurements in the class logsheet. 9. After approximately a half-hour and then again after your last time-point, determine the Fv/Fm of your sample using DCMU (use the 6mL test-tubes). Record the time these measurements were made Determining Fv/Fm using DCMU: There is an alternative procedure for measuring Fv/Fm that only requires a standard bench-top fluorometer. This method uses the chemical DCMU to chemically block the ETC rather than saturating it with light. 2. You will take two reading using this method in addition to using the PAM fluorometer. Materials needed: Diruon minimum 98% (Sigma-Aldrich D2425) a.k.a. 3-(3,4-Dichlorophenyl)-1,1Dimethylurea 6 mL sample of algae 10 mL test tubes Fluorometer 1. 2. 3. 4. 5. 6. Make 10mM stock of DCMU in 90% EtOH Dark adapt cells for 30 minutes in 50 mL amber bottles Eliminate exposure to light as much as possible e.g. dim lights, close shades Read fluorescence (Fo) Add 6 μl DCMU (resulting conc. is 10μM DCMU) Immediately read again (get stable reading) (Fm) Fv/Fm = (Fm-Fo)/Fm Additional features of the PAM fluorometer: Determining contribution of different algal classes The PAM fluorometer is a powerful tool that can make several measurements other than Fv/Fm. It excites the sample with 4 different wavelengths of light. Different accessory pigments will have unique absorption spectra (as you’ve seen with the spectrophotometer). The PAM uses these differences to determine the presence and relative concentrations of 3 different groups of algae in your sample: green algae (by the presence of Chl b), cyanobacteria (by the presence of allophycocyanin), and diatom/dinoflagellate-like algae (by the presence of Chl c, fucoxanthin, & carotenoids). Record measurements below: H2O2: M Time H2O2 added: Timepoint Time Temp: ºC Time in bath: DCMU Measurement 1 Time: F0: FM: Fv/Fm 1 2 3 4 DCMU Measurement 2 Time: F0: FM: 5 6 7 8 9 10 3. Assignment: The combined dataset for all treatments will be posted on the website. If you need help with excel, Sarah will give a tutorial at the end of next week’s lab to anyone who needs it. 1. To look at the effects of temperature alone, plot a graph of time vs. Fv/Fm for the three 0M H2O2 treatments (treatments 1,5, & 9). Did temperature have an affect on Fv/Fm? Temperature Effect (no oxidative stress) 0.6 0.5 0.4 Fv/Fm Here is an example graph of this data done with sigmaplot is. Your excel graph should look similar. 0.3 0.2 0.1 0.0 02:40:00 20º 0uM 35º 0uM 40º 0uM 03:00:00 03:20:00 03:40:00 04:00:00 04:20:00 2. To look at the effect of oxidative stress alone, plot a graph (time vs. Fv/Fm) of the four 20ºC treatments (1 – 4). Did H2O2 cause oxidative stress? At which concentrations? If you were to repeat this experiment, what H2O2 concentrations would you suggest using? 3. Plot the 230M treatments at all three temperatures (3, 7, & 11) on the same graph. On a separate graph, plot the four 40ºC treatments (9 -12). Discuss what these graphs tell you about the interactive effects of temperature stress and oxidative stress on Fv/Fm. 4. Calculate Fv/Fm based on your DCMU measurements. Show your calculations below. Do the Fv/Fm measurements from the two methods agree? If not, what are some possible sources of error? 4.