Casein & Lactose Isolation: Milk Experiment - Lab Manual
advertisement

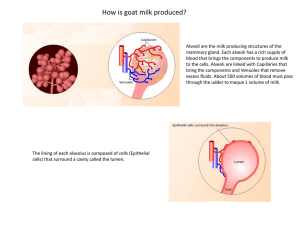
Experiment 5: Isolation of Casein and Lactose from Milk Theoretical Background Milk is the most nutritionally complete food found in nature. All kinds of milk, human or animal, contain vitamins (principally thiamine – VitB1, riboflavin – VitB2, pantothenic acid – VitB5, and vitamins A, B12, D), minerals (calcium,potassium, sodium, phosphorus, and trace metals), proteins(mostly casein), carbohydrates (principally lactose), and lipids (fats). Whole milk is an oil-in-water emulsion, containing its 3.9% fat dispersed as micronsized globules. The fat emulsion is stabilized by complex phospholipids and proteins that are adsorbed on the surfaces of the globules. Because the fat in milk is so finely dispersed, it is digested more easily than fat from any other source. The globules are lighter than water, thus colesce on standing and eventually rise to the surface of the milk as cream. Vitamins A and D are fat-soluble substances and are thus concentrated in the cream. The fats in mil kare primarily triglycerides, which are esters of saturated and unsaturated carboxylic acids with glycerol, a trialcohol [1]. There are three kinds of proteins in milk: casein, lactalbumins, and lactoglobulins. All three are globular proteins, which tend to fold back on themselves into compact, nearly spheroidal units and are more easily solubilizied in water as collodial suspensions than fibrous proteins are. They are “complete proteins”, so called because they contain all the amino acids essential for building blood and tissue, and they can sustain life and provide normal growth even if they are the only proteins, but they can contain greater amounts of amino acids than the proteins in egg and meat [1]. Casein, the main protein in milk, is a phosphoprotein. The phosphate groups are attached to the hydroxyl groups of some of the amino acid side chains. Casein exists in milk as the calcium salt, calcium caseinate. It is actually a mixture of at least three similar proteins which differ primarily in molecular weight and the amount of phosphorus groups they contain ( α, β and κ caseins), they form a micelle, or a solublized unit. Neither the α nor the β casein is soluble in milk and neither is soluble either singly or in combination. If κ casein is added to either one, or to a combination of the two, however, the result is a casein complex that is soluble owing to the formation of the micelle. A structure proposed for casein micelle is shown below [2]: 1 Figure 1. Casein Calcium caseinate has an isoelectric point of pH 4.6. Therefore, it is insoluble in solutions of pH less than 4.6. The pH of milk is about 6.6; therefore, casein has negative charge at this pH and is solubilized as a salt. If acid is added to milk, the negative charges on the outer surface of the casein micelles are neutralized (by protonation of the phosphate groups) and the neutral protein precipitates, with the calcium ions remaining in solution: Ca-caseinate + 2H+ → casein + Ca2+ A natural example of this process occurse when milk sours. The souring of milk is an intricate process started by the action of microorganisms on the principal carbohydrate in milk, lactose. The microorganisms hydrolyse the lactose into glucose and galactose. Once galactose has been formed, lactobacilli, a strain of bacteria present in milk, convert it to the sour-tasting lactic acid. Since the production of the lactic acid also lowers the pH of the milk, the milk clots when it sours due to the precipitation of casein. The structure of lactic acid is shown below [1]: Figure 2. Lactic acid When the fats and proteins have been removed from milk, the carbohydrates remain in the whey, as they are soluble in aqueous solution. The main carbohydrate in milk is lactose. 2 Figure 3. Lactose Lactose (4-O-(β-D-galactopyranosyl)-D-glucopyranose) is the only carbohydrate that mammals synthesize. It is a dissacharide consisting of one molecule of D-glucose and one molecule of Dgalactose joined in 1,4’fashion, and is synthesized in the mammary glands. In this process, one molecule of glucose is converted to galactose and joined to another of glucose [2,3]. Melting Point A melting point can be used to identify a substance and to get an indication of its purity. The melting point (or freezing point) of a solid is the temperature at which the solid exists in equilibrium with its liquid state under an external pressure of one atmosphere. More precisely, it is the temperature at which the vapor pressure of the solid phase becomes equal to the vapor pressure of the liquid phase. Experimentally, it is extremely difficult to establish the exact temperature at which this equilibrium is established; therefore, the temperature range over which liquid and solid are found to coexist is called the melting point. For example, a solid may be reported to have a `melting point' of 100-101oC; this means that, on heating slowly, the first droplet of liquid was observed at 100oC and the last crystal of solid disappeared at 101oC. To determine the melting point of a crystalline substance, a small amount of the finely powdered crystals is introduced into a thin-walled capillary tube; the latter is placed on an electrically heated "hot-stage" and heated. Two temperatures are recorded, the temperature at which the substance begins to liquefy and that at which it becomes completely liquefied. The observed melting point range is the interval between these two temperatures. Several types of apparatus used to determine melting points are shown in Figure 1 [1]. 3 Figure 4. Melting point determination apparatus [1] APPARATUS Equipment Beaker 100 mL * 2 Beaker 25 mL * 2 Erlenmeyer Flask 50 mL Graduated cylinder 10 mL Graduated cylinder 25 mL Filtering Flask Büchner Funnel Thrompt Hot plate Thermometer Dropper Rubber tubing Paper towels Glass rod Chemicals Acetic Acid, CH3COOH Ethyl ether, C4H10O Ethanol, C2H5OH Milk 4 PROCEDURE 1. Measure 50 mL milk by a graduated cylinder and put it in a 100 mL beaker. 2. Heat the solution on a hot plate to bring the temperature to 55°C, control the temperature with thermometer. 3. Prepare 20 mL, 10 % acetic acid solution in a 25 mL beaker. 4. Add dropwise the 10% acetic acid solution while stirring. Do not add the acid too rapidly. 5. Keep the beaker on the hot plate, until the liquid becomes transparent and the casein no longer precipitates. Isolation of Casein 1. Collect the casein with suction filtration and place the casein in a 100 mL beaker. 2. Prepare 20 mL1:1 ethyl ether - ethanol solution in a 25 mL beaker, and then add this mixture to the casein precipitate. Stir the solution for a few minutes. 3. Weigh the fitler paper and collect the casein by suction filtration. 4. Place the casein between several layers of paper towels to dry. 5. Keep the casein and allow it to dry for three days to determine its melting point and calculate the yield of our experiment. Melting Point Determination 1. 2. 3. 4. Fill a melting point tube with the sample (a thin-walled capillary tube) sealed at one end. Attach the capillary tube to a thermometer. Place the capillary in the melting point stage with oil bath (Figure 1.A). turn on the power and allow the hot-stage temperature to rise fairly rapidly to within 15-20oC below the expected melting point of the compound. 5. During the determination of the actual melting point range, heat the melting point hot-stage slowly at a uniform rate, about 2 degrees per minute. 6. Records the temperature at which the substance begins to liquefy and that at which it becomes completely liquefied. 5 EXPECTED RESULTS AND CALCULATIONS Melting point of casein: 280 °C Melting point of casein: 342.3 °C Percentage yield: Percentage of product = amount of product recovered x 100% Weight of milk REPORT OBJECTIVES 1. Calculate the percent yield of your product. Assume the density of milk 1.037 g/cm3. 2. Why do we try to keep the temperature around 55°C? REFERENCES 1. McMaster University, Chem2o6 Lab Manual, 1997, http://www.chemistry.mcmaster.ca/chem2o6/labmanual/ 2. Minard, B., Chem35 Synthetic Experiments, PennState University, 2002 http://courses.chem.psu.edu/chem35/HTML/Experiments/Exp112.pdf 3. Yeditepe University, Department of Chemical Engineering , Bioorganic Chemistry Laboratory Manual, Istanbul, 2006 6