24A-Radioactive Decay Processes - mvhs
advertisement

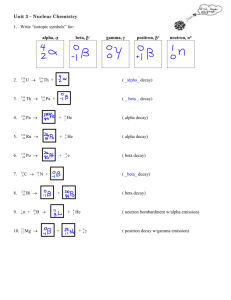
24A-Radioactive Decay Processes Radioactive processes involve the emission or capture of a number of different particles. Many radioactive processes result in the change of either the mass number or the atomic number of the atom undergoing the change. There are two exceptions to this general statement. In the first exception, alpha particle emission, both the atomic number and mass number decrease. When gamma radiation is emitted, neither the atomic number nor the mass number changes. However, the emission of gamma radiation always accompanies another radioactive process. Summary of Typical Radioactive Processes Alpha Mass number (-4), Atomic number (-2) Beta Atomic number (+1) Positron Atomic number (-1) Neutron Mass number (-1) Electron capture Atomic number (-1) In addition to the reactions shown in the table, atoms can be bombarded with alpha particles, neutrons, or other atoms to synthesize different radioisotopes. These reactions are called transmutation reactions. Example A What happens to the mass number and the atomic number of an atom that undergoes beta decay? Solution: In beta decay, a neutron splits into a proton and an electron. The atomic number increases by one; the mass number remains the same. You try it: 1. A radioisotope of an element undergoes alpha particle decay. How does the atomic number and mass number of the particle change? Example B Give the composition of the nucleus of the following isotopes a. 214 At 85 b. 14 C 6 c. 64 Cu 29 Solution: a. 85 protons and 129 neutrons b. 6 protons and 8 neutrons c. 29 protons and 35 neutrons You try it 2. Give the composition of the nucleus of the following isotopes a. 64 Ni 28 b. 136 I 53 c. 195 Au 79 Your Solution Example C Complete each of these equations by writing the correct formula for “?” a. 108 Sn + 0 e ? 50 1 b. 16 N 16 O + 0 e + ? 7 8 -1 c. 89 Kr 88 Kr + ? 36 36 Solution: You try it 3. Complete the following equations for radioactive decay a. 241 Am 4 He + ? 95 2 b. 81 Rb ? + 0 e 37 +1 c. 50 Fe 50 Co + ? 26 27 Your solution: Problems for you to try: 4. Identify the type of radioactive process occurring in each equation in Problem 3 5. Write a nuclear equation for the follow radioactive processes a. alpha decay of francium-208 b. electron capture by beryllium-7 c. beta emission by argon-37 d. positron emission by fluorine-17 6. Complete the equations for these transmutation reactions a. 6 Li + 1 n 4 He + ? 3 0 2 b. 235 U + 1 n ? + 141 Ba + 3 1 n 92 0 56 0 c. 27 Al + 4 He ? + 1 n 13 2 0 d. 235 U 90 Sr + ? + 1 n + 4 0 e 92 38 0 -1 e. 1 n + ? 144 Ce + 90 Sr + 6 1 n + 2 0 e 0 58 38 0 -1 24B-Nuclear Chem Half-life Calculations The decay of a radioactive atom is a random process. Although we can look at the number of protons and neutrons in the nucleus of an atom and predict whether the atom is unstable and therefore will decay, we cannot, however, predict when it will decay. The process of radioactive decay is independent of outside influences like temperature and pressure changes. When we consider a large number atoms of the same radioisotope, we can measure the time it takes for one-half of these atoms to decay and emit radiation experimentally. This measured quantity is called the half-life of the radioisotope. Each radioisotope has a characteristic half-life. The halflives of radioactive isotopes range from very small fractions of a second to millions of years. The half-life of some radioisotopes used in the problems in this worksheet are given in the following table. Radioisotope Cobalt-60 Copper-66 Strontium-90 Palladium-103 Iodine-131 Polonium-214 5.26 years 5.10 minutes 28.8 years 17 days 8.0 days 164 seconds Example A An isotope has a half-life of 3.5 years. How much of a 60.0 g sample of this isotope remains after 7.0 years? Solution: One half the mass will decay is 3.7 years leaving 30.0 g. By the seventh year another one half the sample will decay, leaving 15.0 g. You try it 1. Hydrogen-3 (tritium) has a half-life of 12.3 years. How many years will it take for 88 g of tritium to decay to an 11-gram sample. Your solution: Example B You have 32 million atoms of a radioactive isotope. How many atoms will be left after 5 halflives? Solution: The number of atoms will be halved 5 times. 32 million X .5 X .5 X .5 X .5 X .5 = 1 million atoms. You Try it 2. What fraction of radioactive atoms remain after 7 half-lives? Your solution: Example C Cobalt-60 is used as a source of gamma radiation in treating some canceres. How many years does it take for 50.0 g of this isotope to decay to 6.25 g? Solution: The half-life of cobalt-60 is 5.26 years. It takes 3 half-lives, or 15.8 years to decay to 6.25 g [50.0 g 25.0 12.5 g 6.25 g] You Try it 3. Polonium-214 has a relatively short half-life of 164 s. How many seconds would it take for 8.0 g of this isotope to decay to 0.25 g? Your Solution: Example D Iodine-131 is used for diagnosing and treating Thyroid gland malfunction. How much of a 4.80 gram sample of iodine-131 remains after 32 days? Solution: The half-life of iodine 131 is 8.0 days. Therefore 32 days represents four half lives and 0.3-0 g of the sample remains. [4.8g 2.4 g 1.2 g 0.60 g 0.30 g] You Try it 4. Atmospheric testing of nuclear devices in the 1950’s and the 1960’s resulted in the spread of a number of different radioactive isotopes, including strontium-90. Strontium and calcium are chemically similar and the incorporation of radioactive strontium-90 into the bones of growing children may lead to serious bodily harm. How much of a 56 mg-sample of strontium090 remains in the body after 86.4 years? Your solution: Problems For You To Try: 5. How many days does it take for 16 g of palladium-103 to decay to 1.0 g? 6. By approx. what factor would the mass of a sample of copper-66 decrease in 51 minutes? 7. In 5.49 seconds 1.20 g of argon-35 decays to leave only 0.15 g. What is the half-life of argon35? Activity 22.1-Nuclear Particles 1. Distinguish between the terms nucleon and nuclide. Use examples in your distinction. 2. Describe your understanding of the term radiation. 3. Complete the chart Decay component Mass Charge Alpha Beta Gamma Positron Proton Electron Neutron Notation used Atomic Location 4. a. Which elements are most likely to decay of give off alpha radiation? b. which elements are most likely to decay of give off beta radiation? c. When does the emission of gamma radiation occur? 5. Using your text as a guide, write a balanced nuclear equation for each of the following events. a. alpha emission by 239/93 Np b. beta emission by 30/13 Al c. positron emission by 99/43 Tc d. electron capture by 55/26 Fe e. Beta + emission by 245/95 Am 7. Complete the problem assigned by your instructor. Complete Question 7 with your group after you have each finished answer questions 1-6. Assign each group member a latter: a, b, c, or D. Write in the name of the person assigned to each letter. A________ B_________ C________ D________ 7. The naturally occurring radioactive nuclide U-232 decays through a series of steps. Write the equation for each step as assigned. Determine the nuclide that is the final product of the steps you write. Notice that, except for A, each person will need the result of the previous person’s work. Being with u-232 Person A: alpha, beta, beta Person B: alpha, alpha, alpha Person C: alpha, alpha, Beta, Beta Person D: alpha, beta, beta The final product is_________________. Is this stable? If yes, explain why. If not, explain why not and what would need to happen for it to become stable.