Diffraction of Electrons
advertisement

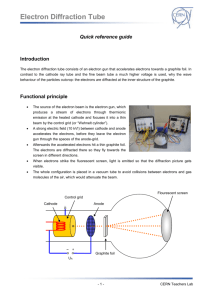
Diffraction of Electrons Purpose The purpose of this lab was to observe electron diffraction and understand the wave properties of electrons. The purpose was also to measure the diffraction effects of electrons as a function of energy and to verify the DeBroglie relation. Introduction Similar to light, electrons also have characteristics of particles and waves. DeBroglie applied the wave theory to electrons and called it the DeBroglie relation. The DeBroglie relation, proved in the 1920’s, is: where p= momentum, h= Plank’s constant, and λ = wavelength. The wavelike character of electrons predicts the energy levels of electrons in simple atoms. The electron has orbits that are orbits of standing waves where only certain wavelengths fit. Atomic spectra are explained by the wave properties of electrons. The energy-momentum relation for non-relativistic elections is substituted into the DeBroglie relation, we get the equation: If E = 2 keV, m = 0.511MeV/c2, h = 4x10-15 eVs, then the wavelength is equal to 0.8 nm. This wavelength is 1,000 times smaller than the wavelength of light; so optical diffraction gratings such as those used in previous lab experiments cannot be used in this experiment. Crystals provide a good grating with a magnitude of 0.2 nm. In this experiment, electrons are produced using a vacuum tube and are accelerated to an energy between 2 keV and 5 keV. The electrons go through polycrystalline graphite. The structure of the crystal is like a hexagon as seen in the figure below. The spacings, d10 and d11, were determined using X-ray diffraction. The polycrystalline sample has crystallites at many different orientations to the incident electron beam. The polycrystalline graphite is in three dimensions. The diffraction peaks, therefore, form concentric circles around the direct beam. These circles are visible because of a phosphor coating on the inside of the cathode ray tube. It was predicted that the wavelength of the electrons would drop and the diameter of the circles would become smaller as the energy was increased. Procedure and Results The ring diameter of the electron diffraction was measured for accelerating voltages between 2.0 kV and 4.0 kV in 0.5 kV intervals. The focus voltage was adjusted as large as possible to get a clear pattern. Calipers were used to measure the diameters in order to minimize electrostatic deflections. The wavelength was calculated using the equation l = dD/2L where d is the spacing, D is the diameter, and L for the Teltron 555 tube was 14.0 cm. The d11 spacing was used with the large diameter calculations and the d10 spacing was used with the small diameter calculations. Using the program, Graphical Analysis, the average wavelength was plotted against the voltage and the graph below was obtained. In order to linearise the plot, the voltage was plotted as V-1/2. The graph of the average wavelength vs. voltage-1/2 is shown below. The measured slope was 1.14 nm. The actual slope comes from the equation: The actual slope is 1.22 nm. The percent error between the lab value and the actual value was 6.56%. The electron diffraction in our experiment was similar to the actual diffraction of electrons. Extracted from: http://www.warren-wilson.edu/~physics/physics2/Formal_2001/JWood/Ediff.htm http://www.google.co.uk/search?q=cache:yD6ovukY5M8J:rustam.uwp.edu/306/bragg.pdf+ele ctron+diffraction+teltron+tube+&hl=cy&lr=lang_en