Electromagnetic Spectrum Basics Assignment
advertisement

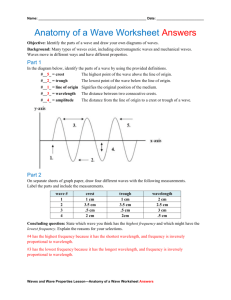
Electromagnetic Spectrum Basics Name ________________________ Period __ Our evidence for electrons and the location of electrons within atoms all comes from evidence in the form of energy emitted by electrons when they absorb energy in the form of radiant energy (like a solar calculator), electricity or are burned in a flame. Energy has both wave and particle properties. As a wave it has wavelength () and frequency (). All electromagnetic radiation travels at the speed of light (C), and the relationship between them is C = Energy behaves as particles in that it exists as photons, discrete packets or bursts of energy. The energy of a photon is proportional to its wave frequency, as determined by Max Planck in his equation E = hwhere h is Planck's constant. C = 3.00 x 108 m/s h = 6.6261 x 10-34 Js 1. Complete the following table: Symbol C h E Name Unit 2. How would each have to change to change yellow light photons into green light photons? : _________________________________________________________________ : _________________________________________________________________ C: _________________________________________________________________ E: _________________________________________________________________ X Y Refer to the electromagnetic waves above to answer the following questions. List ALL correct answers. a. wavelength b. frequency c. speed d. energy e. amplitude ________________ 3. Compared to wave X, wave Y has a greater _________ . ________________ 4. If wave X is green light, it would become more blue if the _________ is decreased. ________________ 5. The brightness, but not the color of light is due to wave _________ . ________________ 6. It is not possible to change the wave ________ of visible light. ________________ 7. Compared to wave X, wave Y has the same ____________ . 8. Find the wavelength of a photon if its frequency is 2.35 x 1016 /s. SHOW WORK using C = 9. Find the energy of a photon if its frequency is 2.35 x 1016 /s. SHOW WORK using E = h 10. Find the frequency of wave X if the wavelength is 5.5 x 10-7 m. SHOW WORK! 11. Find the energy of wave Y if it has a wave frequency of 9.2 x 1014 /s. SHOW WORK! 12. How would the number of photons of light of a certain energy affect the appearance of the light? 13. What form of electromagnetic energy has the lowest frequency? _____________________________ 14. Calculate the wavelength of a photon that has an energy of 2.34 x 10-24 J. Two steps. SHOW WORK! What type of radiation is this? __________________________________________________