Physical Properties of the Universe, Part I: Light
advertisement
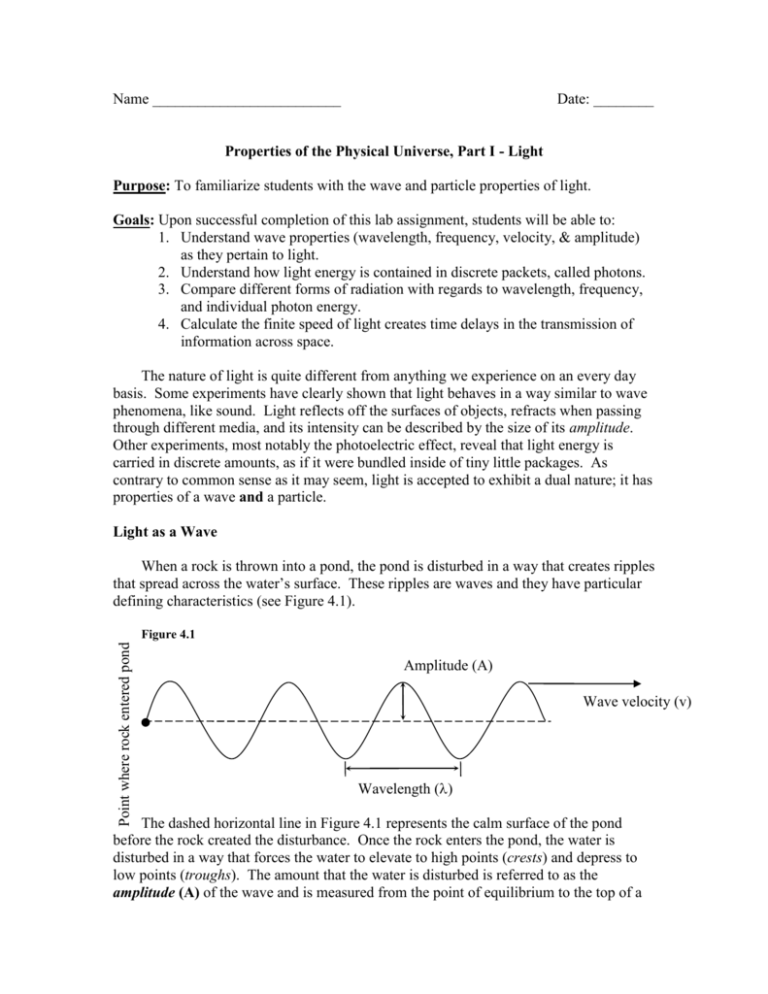
Name _________________________ Date: ________ Properties of the Physical Universe, Part I - Light Purpose: To familiarize students with the wave and particle properties of light. Goals: Upon successful completion of this lab assignment, students will be able to: 1. Understand wave properties (wavelength, frequency, velocity, & amplitude) as they pertain to light. 2. Understand how light energy is contained in discrete packets, called photons. 3. Compare different forms of radiation with regards to wavelength, frequency, and individual photon energy. 4. Calculate the finite speed of light creates time delays in the transmission of information across space. The nature of light is quite different from anything we experience on an every day basis. Some experiments have clearly shown that light behaves in a way similar to wave phenomena, like sound. Light reflects off the surfaces of objects, refracts when passing through different media, and its intensity can be described by the size of its amplitude. Other experiments, most notably the photoelectric effect, reveal that light energy is carried in discrete amounts, as if it were bundled inside of tiny little packages. As contrary to common sense as it may seem, light is accepted to exhibit a dual nature; it has properties of a wave and a particle. Light as a Wave When a rock is thrown into a pond, the pond is disturbed in a way that creates ripples that spread across the water’s surface. These ripples are waves and they have particular defining characteristics (see Figure 4.1). Amplitude (A) Wave velocity (v) Wavelength () pond Point where rock entered pond Figure 4.1 The dashed horizontal line in Figure 4.1 represents the calm surface of the pond before the rock created the disturbance. Once the rock enters the pond, the water is disturbed in a way that forces the water to elevate to high points (crests) and depress to low points (troughs). The amount that the water is disturbed is referred to as the amplitude (A) of the wave and is measured from the point of equilibrium to the top of a crest or the bottom of a trough. The units of measurement will depend on the wave phenomena in question; for a water wave, units of distance are sufficient while the amplitude of sound waves is measured in decibels. The wave made when a rock is thrown into a pond will also travel with a particular velocity (v). Every point on a wave moves with the same speed in a direction that takes it away from the source. In this case, the wave moves away from the point where the rock entered the water while sound waves move away from a set of stereo speakers and light moves away from an illuminated light bulb. The distance between crests (or troughs) is called the wavelength () and it is always measured in units of distance. The wavelength and velocity are related to one another through a quantity called the frequency (f). The frequency is a measurement of how many waves pass a given point every second and is measured in units called Hertz (1 Hz = 1 wave per second). For example, the note ‘middle C’ on a piano is a sound wave with a frequency of 256 Hz. This means that 256 sound waves hit your ear drum every second when the ‘middle C’ key is played. The wavelength and frequency of any given wave are related to the wave’s velocity by the following equation: v = f The speed of sound is treated as a constant measurement (330 m/s), which means that every sound wave has a unique wavelength and frequency (i.e. pitch). Similarly, the speed of light is treated as a constant measurement (3 x 108 m/s) so every light wave will also have a unique wavelength and frequency. Exercises 1. Draw in a double-ended arrow ( ) to indicate the wavelength () and amplitude (A) of each wave in Figure 4.2. Measure the wavelength and amplitude of each wave, in centimeters, and record your results in the table. 2. Calculate the frequency of each wave if they are both moving at the speed of sound (v = 330 m/s). You will need to convert each of your measurements to meters before substituting the values into the wave velocity equation. Table 4.1 Amplitude Wave A Wave B Wavelength Frequency Figure 4.2 Wave A Wave B 3. Calculate the wavelength of a light wave that moves with a velocity of 3.0 x 108 m/s and has a frequency of 5.2 x 1014 Hz. Report your answer in nanometers (nm). 4. What is the frequency of a light wave that has a wavelength of 125 m? Light as a Particle Waves also carry energy. It has been understood for quite some time that the energy that is carried by a given wave is determined by the wave’s amplitude. The more intense the wave, the more energy it carries (think of the damage done along the shoreline by waves during a hurricane as opposed to the waves that normally crash on a beach). Light waves, however, do not behave in this way at all. In order to correctly explain the photoelectric effect, Albert Einstein changed our concept of the very nature of light. The discovery he made, which won him the Nobel Prize, was that light energy is carried in discrete amounts and not as a continual source of energy. When you flip on a light bulb, tiny little packets of light (called photons, see Figure 4.3) come rushing out at the speed of light instead of a single, continuous wave. Figure 4.3: Conceptual drawing of a photon. The energy of an individual photon is dependent upon its wavelength and not its amplitude. The shorter the wavelength of a photon means that more waves can fit into the package, thus the energy will be higher. The energy of a photon depends directly on the photon’s frequency or inversely on the photon’s wavelength according to the relationships below: E photon = hf - or - E photon = hc c speed of light = 3.00 x 10 m/s 8 h Planck' s constant = 6.63 x 10 -34 J s 5. Draw in a double-ended arrow ( ) to indicate the wavelength of each photon in Figure 4.4. Measure each photon’s wavelength and record your results in the table in meters. 6. Calculate the frequency of each photon if they are both moving at the speed of light (v = 3.0 x 108 m/s). You will need to convert each of your measurements to meters before substituting the values into the wave velocity equation. 7. Calculate the energy of each photon using one of the photon energy equations above. Record your answer in the table in units of Joules (J). Figure 4.4 Photon A Photon B Table 4.2 Wavelength Frequency Energy Photon A Photon B Types of Photons: the EM Spectrum All photons move through empty vacuum at the speed of light. This being the case, it is useful to categorize photons into groups according to their wavelength, frequency, and energy. Photons are then often arranged by type according to their wavelengths, from short to long. The boundaries are not strictly defined so a photon may be considered to be “high energy UV” or “low energy X-Ray” depending on the scientist’s interpretation. Figure 4.5 shows how the EM spectrum arranged is from short wavelength to long wavelength. Table 4.3by a table that list one set of accepted wavelength boundaries. Figure 4.5 Gamma Short High f High E Blue X-Rays Green Ultraviolet (UV) Visible Violet Yellow Orange Infrare (IR) Microwave Red Radio Waves Long Low f Low E Table 4.3 Radiation Type Gamma Ray X-Rays Ultraviolet (UV) Visible Light Infrared (IR) Microwaves Radiowaves Wavelength Range Frequency Range Energy Range < 1 pm 1 pm – 1 nm 1 nm – 400 nm 400 – 750 nm 750 nm – 25 m 25 m – 1 mm > 1 mm > 1020 Hz 1020 - 1017 Hz 1017 - 1014 Hz ~ 1014 Hz 1014 - 1013 Hz 1013 - 1011 Hz < 1011 Hz > 10-13 J 10-13 - 10-16 J 10-16 - 10-19 J ~10-19 J 10-19 - 10-21 J 10-21 - 10-22 J < 10-22 J 8. Table 4.4 lists a set of photons of different significance. Complete the table by calculating the wavelength, frequency, and/or energy where necessary. Note that all wavelengths need to be converted to meters before they can be used to calculate any of the other quantities. Table 4.4 Photon Sun Light Human Radiation Microwave Oven Medical X-Rays UV-B Satellite TV Signal Wavelength Frequency Energy 517 nm 3.10 x 1013 HZ 2.45 x 109 Hz 1.00 nm 6.63 x 10-19 J 5.00 x 109 Hz Speed of Light It may seem instantaneous for light to travel across the room when we flip on a light switch, but light travels at such a high speed that it takes an unperceivable amount of time to travel across a tiny room that our eyes cannot perceive the time delay. Though the speed of light is the fastest that anything in the universe can travel, it is possible to measure it to high accuracy using sophisticated lab equipment. Currently, the speed of light is measured to be 299,792,458 m/s, though in this course it will be sufficient to use the rounded version of the number: c = 3.00 x 108 m/s. There are a couple of things to keep in mind about the speed of light. - Only light can travel at the speed of light. Anything with a mass, no matter how small, CANNOT achieve the speed of light (though some tiny particles can get really close). - When talking about the speed of light we can use either the wave model or the photon model. In the wave model, we can think of the entire wave moving at the speed of light whereas in the photon model we think of the packet of energy moving at that speed. The reason that a photon packet can move at the speed of light is because the packet is massless being that it is pure energy. - The speed of light is constant regardless of the frame of reference. This empirical fact of nature has some strange, and possibly useful, consequences that we will explore in a later lab. - All forms of light will move at the same speed in vacuum. In other words, visible light from the Sun, x-rays from a distant stellar explosion, and radio waves being broadcast by a possible alien civilization all travel through empty space at the same 3.00 x 108 m/s. However, the speed of light will slow down to different degrees once it travels through any substance (air, water, glass). Since so much of our information is transmitted through various forms of light, it is important to understand that there is a time delay between when information is given out vs. when the information is received. The fact that the speed of light is constant as it travels through space is critical when computing these delay times. Below are several examples where it is useful to understand how long delay times are. Determine the delay times in each of the cases below using the relation: c = d t 9. A scientist operating the Hubble Space Telescope wants to look at a new object. In order to reposition the telescope, the controller needs to send a radio signal to the Hubble, which is orbiting the Earth at an elevation of 560 km. Calculate how long it takes a for the radio signal to get from the command center to the telescope. 10. If the Sun were to blow up, we would not know it instantly because it takes time for the light from the explosion to reach Earth. Calculate how long after the explosion occurs we would receive its light given the distance to the Sun is 150 million km. 11. When trying to communicate with any of the spacecraft or rovers on Mars, the operators have to consider the time delay in the transmission when sending commands. Calculate the time delay that would be expected when a command is sent to a rover on Mars when Mars is at a distance of 76.5 x 106 km. Reflection Light will interact with matter in ways that are explained on a macroscopic and microscopic level. In this section we will focus on one macroscopic interaction (reflection) while saving the microscopic ones for when we discuss the quantum nature of matter. When light encounters an object, the object will absorb some of the light while the rest of the light will reflect, or bounce, off of the surface. Different amounts of incident light will reflect depending on the properties of the object, namely density and composition. As a simple example, think of how a mirror made of glass produces a better reflection than a wooden tabletop. Scientists can measure the fractional amount of light that reflects off of an object and interpret that amount as an indication of the object’s composition. Such a measurement is referred to as the object’s albedo and is reported as a decimal value between 0 and 1.00 (corresponding to 0% to 100%). 12. Below is a table of measured albedos for different objects/substances: Object/Substance Albedo Grass Forest Granite Sand Ice Soil Snow 0.05 – 0.30 0.05 – 0.10 0.30 – 0.35 0.20 – 0.40 0.60 0.05 - 0.30 0.80 – 0.90 Based on the information in the above table, would scientists be better served to look for a world with a low albedo or a high albedo if they were interested in detecting possible life on that world? Explain your reasoning.