Electrical Properties of Materials
advertisement
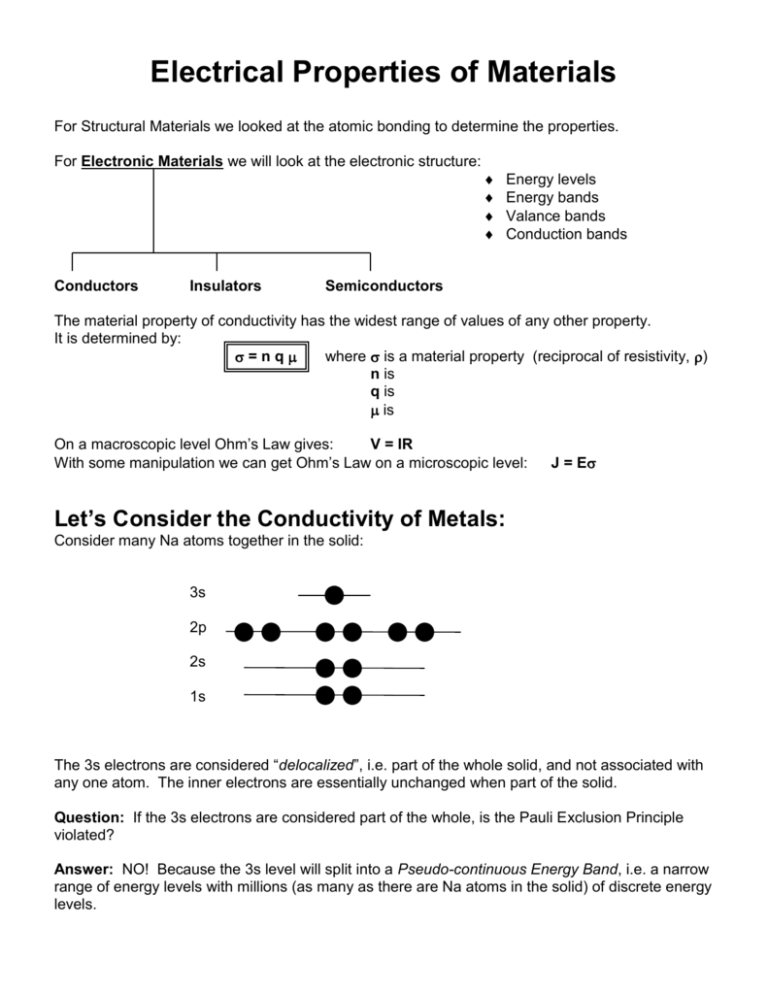
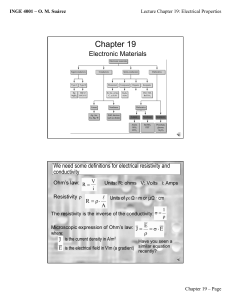
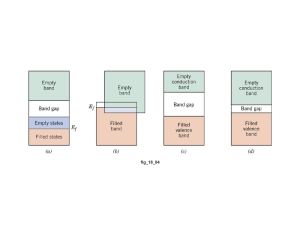
Electrical Properties of Materials For Structural Materials we looked at the atomic bonding to determine the properties. For Electronic Materials we will look at the electronic structure: Conductors Insulators Energy levels Energy bands Valance bands Conduction bands Semiconductors The material property of conductivity has the widest range of values of any other property. It is determined by: =nq where is a material property (reciprocal of resistivity, ) n is q is is On a macroscopic level Ohm’s Law gives: V = IR With some manipulation we can get Ohm’s Law on a microscopic level: J = E Let’s Consider the Conductivity of Metals: Consider many Na atoms together in the solid: 3s 2p 2s 1s The 3s electrons are considered “delocalized”, i.e. part of the whole solid, and not associated with any one atom. The inner electrons are essentially unchanged when part of the solid. Question: If the 3s electrons are considered part of the whole, is the Pauli Exclusion Principle violated? Answer: NO! Because the 3s level will split into a Pseudo-continuous Energy Band, i.e. a narrow range of energy levels with millions (as many as there are Na atoms in the solid) of discrete energy levels. Note: Hunds Rule says that each level will be half filled. This is the Valance Band because it contains the valance electrons. There is high mobility in this band because the energy levels are so close. Hence n is large and is high high value of conductivity, The electron energy level picture for metals looks like: Valance Band (partially filled) This is also the Conduction Band Forbidden Energies Inner Energy levels (completely filled) Now let’s Consider the Conductivity of Insulators: Consider Carbon: diamond cubic structure valance electrons are all covalently bonded in sp3 orbitals the next available energy level is 6eV above the highest occupied level Conduction Band is empty (if electrons could get here, they would conduct) Eg = 6eV Forbidden Energies Valance Band is completely filled n = 0 and so conductivity is virtually zero Now let’s Consider the Conductivity of Semiconductors: Consider Silicon: diamond cubic structure valance electrons are all covalently bonded in sp3 orbitals the next available energy level is 1.1eV above the highest occupied level Conduction Band is not empty because the energy gap is such that at room temp some electrons can be promoted into it, leaving holes in the valance band! Eg = 1.1eV Forbidden Energies Valance Band is completely filled until electrons are promoted into the CB leaving holes here. There are two types of carriers for electrical conduction: electrons, ne and holes, nh (ne = nh) = neqe + nhqh = niq(e + h) where i stands for intrinsic carrier concentration For Semiconductors Eg < 2eV and for Insulators Eg > 2eV Now let’s Consider the effect of adding impurities or increasing temperature on the conductivity of metals and semiconductors Metals Adding impurities or increasing temperature will not change n or q, but both will decrease mobility. (Why?) Hence, conductivity of metals will decrease as temperature increases. Hence, conductivity of metals will decrease as amount of impurities increase. Alternatively, resistivity of metals will increase as temperature increases. Alternatively, resistivity of metals will increase as amount of impurities increase. The relationship of Temperature with resistivity is easier to see because it is linear: = roomTemp[1 + (T - TroomTemp)] where = temperature coefficient of resistivity = o[1 + x] where x is the % of impurities and o is the resistivity at 0% impurities and is a constant for a given system Semiconductors As temperature increases: mobility will decrease (as with metals) q does not change n will increase exponentially since promoting electrons to the CB is a T.A.P. ni = C e – (Eg – Eave)/kT = C e – (Eg / 2kT) and since this will dominate the conductivity equation: = C e – (Eg / 2kT) log slope = – (Eg / 2k) 2.3 1/T Now consider adding impurities: When we add impurities to semiconductors we call them dopants and the process is called doping. The result is a dilute (100 -1000 ppm) substitutional solid solution. There are two kinds of dopants: one will give negative charge carriers (to make an n-type semiconductor) and the other will give positive charge carriers (to make a p-type semiconductor). n-type semiconductors add VA such as P+5 donor impurity atoms in substitutional solid solution with Si the extra valance electron is only loosely bound to the P atom in a donor energy level the binding energy of this electron is approx. 0.4eV and so it is easy to promote it to the CB CB Ec Ed log intrinsic range E = Ec -Ed exhaustion range Eg extrinsic range Ev VB slope = -(Ec -Ed)/2.3k 1/T =nq As T increases, n (the electrons in the Conductance Band) increases exponentially and so dominates in this equation. = oe – (Ec -Ed) / kT After these electrons are all promoted to the Conductance Band, (i.e. are exhasted,) there is a range of temperatures before intrinsic semi-conduction kicks in where the conductivity remains essentially constant. After that, as temperature increases, there will be a promotion of electrons from the VB into the CB. (intrinsic behavior) p-type semiconductors add IIIA such as B+3 donor impurity atoms in substitutional solid solution with Si the lack of an electron needed for sp3 tetrahedral bonding is easily filled by a neighboring Si atom into an acceptor energy level, Ea Ea is only slightly above the VB and so it is easy to promote an electron from the VB into it log CB Ec intrinsic range saturation range Eg Ea Ev extrinsic range E = Ea –Ev slope = -(Ea –Ev)/2.3k VB 1/T =nq As T increases, n (the holes in the Valence Band) increases exponentially and so dominates in this equation. = oe – (Ea -Ev) / kT After these electrons are all promoted to the acceptor level, (i.e. this level is saturated,) there is a range of temperatures before intrinsic semi-conduction kicks in where the conductivity remains essentially constant. After that, as temperature increases, there will be a promotion of electrons from the VB into the CB. (intrinsic behavior)

![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)