What you need to know from Chapter 4: (review the on
advertisement

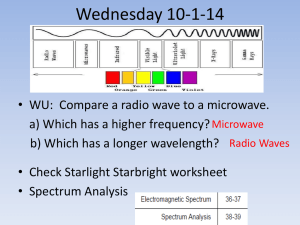
What you need to know from Chapter 4: (review the on-line notes for more detailed explanation) -what are the properties of light? -speed, wavelength, frequency, energy, and intensity --> speed is constant in a vacuum --> energy increases as frequency increases --> energy increases as wavelength decreases --> intensity is equivalent to brightness ===> therefore, the color of the light indicates whether it has high energy or low energy: blue light (short-wavelength, high frequency) has more energy than red light (long-wavelength, low frequency). The colors of the rainbow (or visible spectrum, the light we can see with our eyes) in order of low energy to high energy are: Red, Orange, Yellow, Green, Blue, Indigo, Violet (low energy) -----------------------------------> (high energy) R O Y G B I V -visible light (what we see with our eyes) is only a tiny portion of the full electromagnetic (E/M) spectrum --> you should know the names of the other regions (gamma-rays, x-rays, etc.; see the class notes or text) and be able to put them in order from low energy to high energy. --> why is it important to study the regions of the electromagnetic spectrum that we can't see? --> different physical processes produce light in different parts of the E/M spectrum; we can't get a complete understanding of an astronomical object (star, planet, or galaxy for instance) by studying the light emitted in only one part of the E/M spectrum --> an object that emits most of it's energy in radio waves (cool gas throughout or surrounding a galaxy) for instance, may emit little or no visible light; we could not detect it without radio telescopes -light can be described like a wave or a particle (called a photon) (see notes 09_PSC1010-em_spectrum.ppt) Energy: --> long wavelength, or (low frequency) low energy light corresponds to low energy photons --> short wavelength or (high frequency) high energy light corresponds to high energy photons Intensity: --> high intensity (bright) light corresponds to high photon flux, or many photons received from a light source --> low intensity (faint) light corresponds to low photon flux, or few photons received from a light source What is a spectrum? (see notes 10_PSC1010-atomic_structure.ppt) --> it shows the relative brightness of the different energy regions of the electromagnetic spectrum --> considering only the light we can see for example, a visible light spectrum is the colors of the rainbow, which are always (in nature) in this order from low to high energy: red, orange, yellow, green, indigo, violet. --> the visible light spectrum of a cool star would show a rainbow that was bright on the red (low energy) end and faint on the blue (high energy) end --> conversely, the visible light spectrum of a hot star would show a rainbow that was bright on the blue (high energy) end and faint on the red (low energy) end --> of course, some objects may emit most of their light in regions of the electromagnetic spectrum that we can't see, like shocked (compressed at high speed) gas (primarily X-rays; high-energy) or electrons trapped in the magnetic field (radio waves; low-energy) of a neutron star --> special telescopes and detectors (cameras) must be built to study the light from these objects What are the types of Spectra? 1) Continuous spectrum - light from a solid object or dense gas, glows with light corresponding to its temperature [for example, the light from a star] -->see slides on Wien's law -hot objects emit more intense (brighter) high energy light (cool stars are red) -cool objects emit more intense (brighter) low energy light (hot stars are blue) --> note also from the Wien's law plots that for stars of the same size a hot star will be brighter than a red star: in the plot, the peak of the blue-star-spectrum is higher than the peak of the red-star-spectrum, therefore, the blue star is brighter 2) Absorption spectrum - produced when observing a cooler gas in front of a hotter star [for example, a gas cloud in front of a star] --> produces dark lines in the spectrum, also called absorption lines --> these occur because atoms in the gas absorb the light from the star, but only at specific, discrete wavelengths (or energies) --> if the light is absorbed by the gas, we can not see that light, therefore the spectrum is dark at those specific, discrete wavelengths --> they are discrete because the electrons in the atoms in the gas are only permitted to have specific, discrete energy levels --> the absorbed light gives energy to (or excites) the electrons in the atoms in the gas and raises the electrons to higher energy levels 3) Emission spectrum - produced when observing a hot, rarified (not dense) gas [for example, the gas leftover from a supernova, also called a supernova remnant] --> produces bright lines in a spectrum, also called emission lines --> these are produced at specific, discrete wavelengths when an electron drops from a high energy level to a low energy level What can we learn by studying light from an astronomical object: From emission or absorption spectra: 1) what chemical elements are present in the object producing the spectrum --> The electrons in each type of atom (hydrogen, helium, carbon, nitrogen, oxygen, etc.) have different and unique energy levels. Therefore, the absorption and/or emission lines in a gas are like unique fingerprints for the atoms in the gas. We know from atomic and quantum theory (and lab measurements) what those energy levels are, so observing what lines are present in an absorption or emission spectrum, we can determine what elements are in the gas that produced the spectrum. 2) what the temperature, density, and abundance of the gas producing the spectrum --> The strength, or brightness of the emission lines, or faintness of the absorption lines can give us the temperature and density of the gas, and how much of the different atoms are present in the gas (abundance). 3) the line of sight velocity of the gas or star (is it moving toward or a away from us and how fast) --> lines are shifted to low energy (red-shifted) for objects moving away from us --> lines are shifted to high energy (blue-shifted) for objects moving toward us ===> by comparing the observed position of the spectral lines from a moving object with their known positions from an at-rest (not-moving) object (from lab measurements or quantum theory) we can find out if the object is moving toward or away from us and at what speed From a continuous spectrum: 4) we can find its temperature (Wien's law for solids or dense gases: hot objects are blue, cool objects are red) From images: 5) we can find its position and its brightness in whatever energy band we are observing (radio, infrared, visible, ultraviolet, x-ray, or gamma-ray)