Thermodynamic Properties in HYSYS for the Peng
advertisement
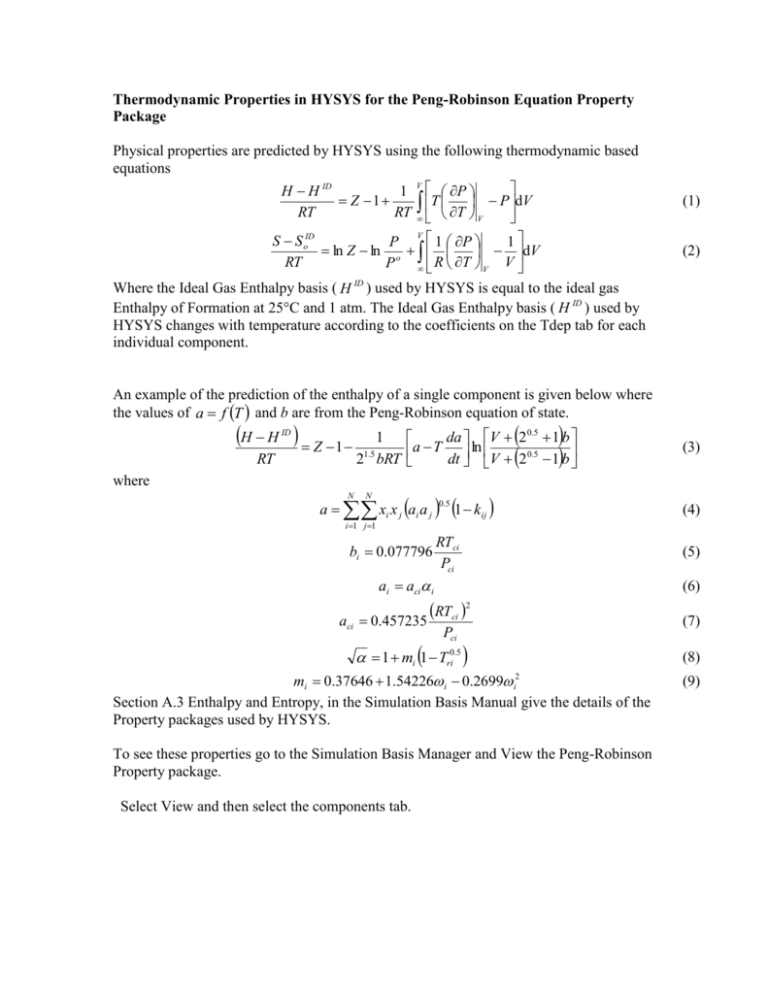
Thermodynamic Properties in HYSYS for the Peng-Robinson Equation Property Package Physical properties are predicted by HYSYS using the following thermodynamic based equations V H H ID 1 P Z 1 T P dV RT RT T V V ID 1 P S So P 1 ln Z ln o dV RT R T V V P Where the Ideal Gas Enthalpy basis ( H ID ) used by HYSYS is equal to the ideal gas Enthalpy of Formation at 25°C and 1 atm. The Ideal Gas Enthalpy basis ( H ID ) used by HYSYS changes with temperature according to the coefficients on the Tdep tab for each individual component. (1) (2) An example of the prediction of the enthalpy of a single component is given below where the values of a f T and b are from the Peng-Robinson equation of state. H H Z 1 ID RT 1 1.5 2 bRT da V 2 0.5 1 b a T dt ln V 2 0.5 1 b (3) where a xi x j ai a j 1 kij N N 0.5 (4) i 1 j 1 bi 0.077796 RTci Pci ai aci i aci 0.457235 (6) RTci 2 Pci 1 mi 1 Tri0.5 mi 0.37646 1.54226 i 0.2699 i2 Section A.3 Enthalpy and Entropy, in the Simulation Basis Manual give the details of the Property packages used by HYSYS. To see these properties go to the Simulation Basis Manager and View the Peng-Robinson Property package. Select View and then select the components tab. (5) (7) (8) (9) Select a component and look at the properties. These are snapshots from the TDep tab or Temperature Dependent properties. Note for volumetric flows: (See Simulation Basis A.5.2) The volumetric flow rate reference state is defined as 60°F and 1 atm when using Field units or 15°C and 1 atm when using SI units.











