Nuclear Chemistry Practice Test Multiple Choice: Identify the choice
advertisement

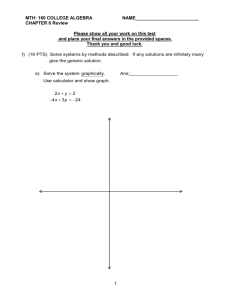
Nuclear Chemistry Practice Test Multiple Choice: Identify the choice that best completes the statement or answers the question. ____ 1. In nuclear chemistry, an atom is referred to as a(n) a. nuclide. b. nucleon. c. nucleus. d. alpha particle. ____ 2. What does the 101 in represent? a. the mass number b. the atomic number c. the nuclide number d. the number of neutrons ____ 3. The nuclear binding energy is released when a nucleus a. is bombarded. c. is formed from its constituent particles. b. divides. d. decays. ____ 4. Balance the following equation: ____ a. b. c. d. ____ ____ ____ ____ ____ ____ ____ 5. Balance the following equation: a. b. + ____ 6. Balance the following equation: a. b. + c. d. + ____ c. d. 7. The spontaneous disintegration of a nucleus into a slightly lighter and more stable nucleus, accompanied by emission of particles, electromagnetic radiation, or both, is a. nuclear fusion. b. nuclear radiation. c. nuclear decay. d. nuclear fission. 8. During radioactive decay, the nucleus disintegrates into a. a lighter and more stable nucleus. c. a lighter and less stable nucleus. b. a heavier and more stable nucleus. d. a heavier and less stable nucleus. 9. Which of the following radioactive decay processes does not reduce the atomic number of a nuclide? a. alpha decay c. both alpha & beta decay b. beta decay d. neither alpha nor beta decay 10. Beta particles are a. electrons. b. helium nuclei. c. pure energy. d. neutrons. 11. Gamma rays are a. electrons. b. helium nuclei. c. pure energy. d. neutrons. ____ 12. The half-life of an isotope is the time required for half the nuclei in a sample to undergo___. a. radioactive decay b. nuclear fission c. nuclear fusion d. chemical changes ____ 13. What is the half-life of an isotope if 125 g of a 500 g sample of the isotope remain after 3.0 years? a. 1.5 years b. 2.5 years c. 3.5 years d. 4.5 years ____ 14. Which is not a parent nuclide? a. uranium-238 b. lead-206 c. uranium-235 d. thorium-232 ____ 15. A decay series ends with a____. a. stable nuclide. b. parent nuclide. c. fission reaction d. beta decay. ____ 16. Artificial radioactive nuclides are produced by a. bombarding stable nuclei with particles. b. using an accelerator to overcome the nuclear force. c. beta emission. d. fission of stable nuclides. ____ 17. How are elements artificially transmuted? a. Stable nuclei are bombarded with charged particles. b. Stable nuclei are bombarded with uncharged particles. c. Stable nuclei are bombarded with charged and uncharged particles. d. Unstable nuclei are bombarded with charged and uncharged particles. ____ 18. Which of the following has the greatest penetrating ability? a. alpha particles b. beta particles c. gamma rays d. All are equal. ____ 19. What are often used to monitor the approximate radiation exposure of people working with radioactive materials? a. film badges c. scintillation counters b. X-ray films (radiographs) d. radioactive tracers ____ 20. Which of the following processes produces nuclei of lower mass than the reactants? a. fission b. fusion c. Both a and b d. Neither a nor b ____ 21. Which are not products of the fission of uranium? a. neutrons b. medium-weight nuclei c. energy d. alpha particles ____ 22. Which of the illustrations below represents a fission reaction? a. A b. B c. C d. D Short Answer 23. Explain the difference between a nuclear power plant and a nuclear bomb. 24. Briefly describe alpha particles, beta particles, and gamma rays. 25. Explain how a chain reaction occurs. Problem 26. Write the nuclear equation for each of the following reactions. Refer to a periodic table. a. the alpha decay of b. the beta decay of c. the decay of Po-210 if it undergoes 2 consecutive alpha decay followed by a beta decay followed by another alpha decay. 27. What is the half-life of an isotope if after 2.00 weeks you have 58.4 g remaining from a 200.0 g starting sample size? 28. Phosphorus-32 has a half-life of 14.3 days. How many milligrams of phosphorus-32 remain after 61.0 days if you start with 4.00 mg of the isotope? Essay 29. Radioisotope Half-life Radon-222 3.82 days Iodine-131 8.07 days Carbon-14 5730 years Thorium-230 75,200 years Uranium-238 4.47x109 years a. From the above list of radioactive elements, select the one best suited to radioactively date an object believed to be about 200,000 years old. b. Explain how you made your choice Nuclear Chemistry Practice Test Answer Section MULTIPLE CHOICE 1. ANS: A OBJ: 1 2. ANS: B OBJ: 1 3. ANS: C OBJ: 2 4. ANS: A OBJ: 4 5. ANS: B OBJ: 4 6. ANS: B OBJ: 4 7. ANS: C OBJ: 1 8. ANS: A OBJ: 1 9. ANS: B OBJ: 2 10. ANS: A OBJ: 2 11. ANS: C OBJ: 2 12. ANS: A OBJ: 3 13. ANS: A Solution: PTS: STA: 14. ANS: OBJ: 15. ANS: OBJ: 16. ANS: OBJ: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: 1 DIF: I SC.A.2.4.3 1 DIF: I SC.A.2.4.3 1 DIF: I SC.A.2.4.3 | SC.C.2.4.4 1 DIF: II SC.A.2.4.3 1 DIF: II SC.A.2.4.3 1 DIF: II SC.A.2.4.3 1 DIF: I SC.A.2.4.3 | SC.C.2.4.4 1 DIF: I SC.A.2.4.3 | SC.C.2.4.4 1 DIF: I SC.A.2.4.3 1 DIF: I SC.A.2.4.3 1 DIF: I SC.A.2.4.3 1 DIF: I SC.A.2.4.3 | SC.C.2.4.4 1 DIF: III SC.A.2.4.3 | SC.C.2.4.4 B PTS: 1 4 STA: SC.A.2.4.3 A PTS: 1 4 STA: SC.A.2.4.3 A PTS: 1 5 STA: SC.A.2.4.3 REF: 1 REF: 1 REF: 1 REF: 1 REF: 1 REF: 1 REF: 2 REF: 2 REF: 2 REF: 2 REF: 2 REF: 2 REF: 2 OBJ: 3 DIF: II REF: 2 DIF: I REF: 2 DIF: I REF: 2 17. ANS: OBJ: 18. ANS: OBJ: 19. ANS: OBJ: 20. ANS: OBJ: 21. ANS: OBJ: 22. ANS: OBJ: C 5 C 1 A 3 A 1 D 1 D 1 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: 1 DIF: I REF: SC.A.2.4.3 1 DIF: I REF: SC.C.2.4.4 1 DIF: I REF: SC.A.2.4.3 | SC.H.3.4.6 1 DIF: I REF: SC.A.2.4.3 | SC.A.2.4.4 | SC.C.2.4.4 1 DIF: I REF: SC.A.2.4.3 | SC.A.2.4.4 | SC.C.2.4.4 1 DIF: II REF: SC.A.2.4.3 | SC.A.2.4.4 | SC.C.2.4.4 2 3 3 4 4 4 SHORT ANSWER 23. ANS: Both involve nuclear fission, but a nuclear power plant takes steps to control the reaction, whereas a bomb is an uncontrolled reaction. PTS: 1 DIF: II REF: 2 OBJ: 1 STA: SC.A.2.4.3 | SC.C.2.4.4 24. ANS: Alpha particles are helium nuclei that are emitted from heavy elements. Beta particles are high-energy electrons emitted from nuclei when neutrons become protons. Gamma rays are high-energy electromagnetic waves. PTS: 1 DIF: II REF: 2 OBJ: 2 STA: SC.A.2.4.3 25. ANS: Neutrons produced by a nuclear reaction can initiate the same reaction in surrounding nuclides, producing more neutrons to initiate more reactions. PTS: 1 DIF: II REF: 4 STA: SC.A.2.4.3 | SC.A.2.4.4 | SC.C.2.4.4 PROBLEM 26. ANS: a. b. c. OBJ: 1 PTS: 1 STA: SC.A.2.4.3 27. ANS: Solution: DIF: II REF: 1 OBJ: 4 PTS: 1 DIF: III STA: SC.A.2.4.3 | SC.C.2.4.4 28. ANS: 0.125 mg Solution: REF: 2 OBJ: 3 PTS: 1 DIF: III STA: SC.A.2.4.3 | SC.C.2.4.4 REF: 2 OBJ: 3 ESSAY 29. ANS: The radioactive isotopes in a material decay over time. If the half-life of an isotope and the original amount of the isotope are known, the age of the material containing the isotope can be estimated. PTS: 1 STA: SC.H.3.4.6 DIF: II REF: 3 OBJ: 4