Physics Active Learning (PAL)
advertisement
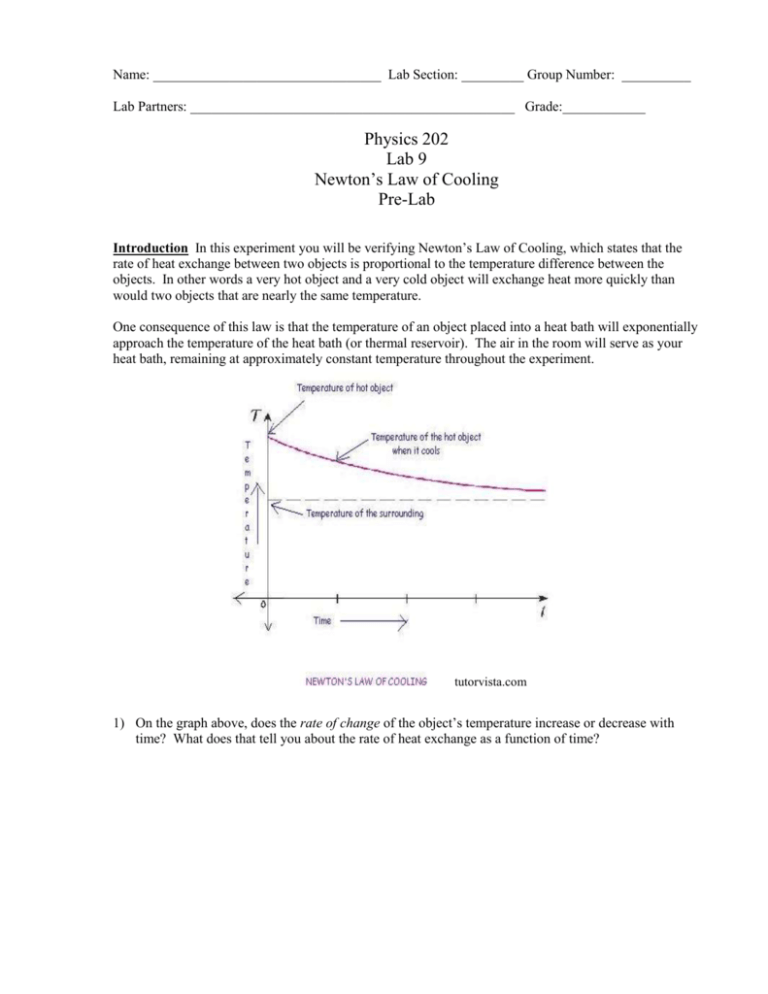
Name: _________________________________ Lab Section: _________ Group Number: __________ Lab Partners: _______________________________________________ Grade:____________ Physics 202 Lab 9 Newton’s Law of Cooling Pre-Lab Introduction In this experiment you will be verifying Newton’s Law of Cooling, which states that the rate of heat exchange between two objects is proportional to the temperature difference between the objects. In other words a very hot object and a very cold object will exchange heat more quickly than would two objects that are nearly the same temperature. One consequence of this law is that the temperature of an object placed into a heat bath will exponentially approach the temperature of the heat bath (or thermal reservoir). The air in the room will serve as your heat bath, remaining at approximately constant temperature throughout the experiment. tutorvista.com 1) On the graph above, does the rate of change of the object’s temperature increase or decrease with time? What does that tell you about the rate of heat exchange as a function of time? 2) On the graph above, does the temperature difference between the object and its surroundings increase or decrease with time? Using this answer and your answer to (1), what can you conclude about the rate of heat exchange as a function of temperature difference? Does this agree with Newton’s Law of Cooling as stated in the first paragraph above? Consider two friends who meet at a coffee shop. Both order hot coffee and both want to add some almond-flavored cream (which is cool) to their hot coffee. They know that they will be so busy talking that they probably will not drink their coffees for half an hour, but they would like the coffee to still be as hot as possible when they drink it. One friend decides to add the cream to her coffee immediately, figuring that, if she cools the coffee right away, it will lose heat at a slower rate during the half-hour of chatting, and thus will be hotter in the end. The other friend decides to wait until just before she drinks the coffee to add the cream, figuring that adding cool cream to cooler coffee will make less of a temperature change in the coffee, and thus it will be hotter in the end. 3) Which friend is right? Which coffee will be hotter when they quit talking and start drinking? Why? Name: _________________________________ Lab Section: _________ Group Number: __________ Lab Partners: _______________________________________________ Grade:____________ Physics 202 Lab 9 Newton’s Law of Cooling Introduction In this experiment you will be verifying Newton’s Law of Cooling, which states that the rate of heat exchange between two objects is proportional to the temperature difference between the objects. In other words a very hot object and a very cold object will exchange heat more quickly than would two objects at nearly the same temperature. One consequence of this law is that the temperature of an object placed into a heat bath will exponentially approach the temperature of the heat bath (or thermal reservoir). The air in the room will serve as your heat bath, remaining at approximately constant temperature throughout the experiment. tutorvista.com IMPORTANT NOTES TO KEEP IN MIND: *You will be taking temperature readings of a cup of hot water. Whenever you read the temperature, make sure the thermometer probe is under water, and not touching the aluminum cup. *Boiling water is very hot. Be careful not to burn yourself. Procedure, Part 1: Heat water in the microwave until it is close to boiling (at least 85o C). In the meantime, measure the mass of the aluminum cup, and rinse it with warm water, so that it will be warm and not cool your hot water too much. Fill the cup about 1/2 full of almost-boiling water, recording the total mass of the cup and water very quickly as you do so. Cover the cup, and immediately begin taking temperature readings every 30 seconds for 30 minutes. Fan the cup continuously during this time, and stir the water occasionally to avoid hot/cool spots. 1) Why do you fan the cup? What will happen if you don’t? How will that change your results? After 30 minutes of temperature readings add an accurately measured amount of cold water from the refrigerator (25-30 g), cover and stir. Immediately measure the temperature to get a final temperature for part 1. 2) Graph the temperature of the water as a function of time, excluding the final temperature reading. Does the graph look exponential? Does the exponential function seem to be approaching zero or some other value? Is this as expected? Fit the data to an exponential, recording all the fit data and errors. Procedure, Part 2: You will be repeating the above experiment, with the same mass of water, and the same initial temperature, and adding the same mass of refrigerated water (in all cases as close to the same as practicable). The difference will be that this time you will add the cold water at t=0 instead of waiting until the end. Measure the temperature immediately before and immediately after adding the cold water, and then begin temperature readings every 30 seconds for 30 minutes, using the same techniques as in part 1. 3) What is the final temperature in this case? Compare it to the final temperature in part 1. Which final temperature is higher? 4) Does this agree with your prediction in the pre-lab about whose coffee will be hotter in the end? Based on your results, which is the better strategy – adding the cream to your coffee immediately or waiting until just before you drink it? Post-lab Questions: 1) Consider the fit of your graph. Your data was fit to the function y=A1e-x/t1+y0. a. What was your value for y0? What physical property does this represent? What would you change about the experimental setup to get a different value of y0? b. What was your value for A1? What physical property does this represent? What would you change about the experimental setup to get a different value of A1? c. What was your value for t1? What physical property does this represent? What would you change about the experimental setup to get a different value of t1? 2) What was the objective of this lab? Do you feel the objective was appropriately achieved? Why or why not? 3) How much scatter (in oC) did your data points have from the fit curve? Do you think this is due mostly to limitations of the accuracy of the thermometer? If not, what do you were the major causes of this scatter?
![저기요[jeo-gi-yo] - WordPress.com](http://s2.studylib.net/store/data/005572742_1-676dcc06fe6d6aaa8f3ba5da35df9fe7-300x300.png)