Word file (281 KB )
advertisement

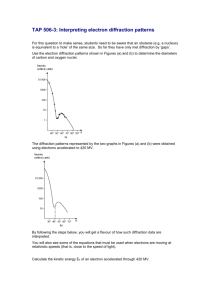
Supplementary Materials Methods As starting materials we used samples of iron (99.999% purity), silicon (99.999% purity), and amorphous silica (99.99% purity). Iron-silicon alloys were synthesized by arc melting appropriate amounts of silicon and iron rod in an arc furnace in a pure argon atmosphere. The samples were powdered by milling and then homogenized in vacuum at 900°C for at least 120 hours. Experiments in multianvil apparatus were performed in a 1200-tonne press. The sample assembly consisted of an MgO (+5 wt% Cr2O3) octahedron (18 mm edge length) containing a LaCrO3 heater. The octahedron was compressed using 32 mm tungsten carbide anvils with a truncation edge length of 11 mm and pyrophyllite gaskets. Temperature was monitored with a W3Re/W25Re thermocouple located axially with respect to the heater and with a junction in a direct contact with the MgO capsule. The P-T uncertainties are estimated to be 1 GPa and 50 K, respectively. The sample (mixture of Fe (80 wt%), Si (5 wt%), and SiO2 (15 wt%)) was contained in MgO capsule, which reacted during the experiments forming magnesiowüstite. In each experiment the sample was first compressed to the desired pressure; the temperature then raised with 100 K/min to the desired run temperature and was then held there for 60 – 90 min. The sample was finally quenched by switching off the power to the furnace and then slowly decompressed. After the completion of each experiment, the entire sample assembly was mounted in epoxy, sectioned through the centre of the sample and then polished. One part was used for the electron microprobe analysis and another part served as a source of material for DACs experiments. Chemical analysis was done with the CAMECA SX 50 electron microprobe. The details of the experiments performed with electrically- and laser-heated DAC are described in our earlier papers1–3. At ESRF powder diffraction experiments were conducted at the beam lines BM01 and ID30. At the BM01 beam line the data was collected with the MAR345 detector using an X-ray beam of 0.6996 Å wavelengths and a size of 50x50 m (beam line BM01), and at ID30 we used MAR345 or Bruker CCD area detectors and a highly focused beam of 10x15 m. The detector-to-sample distance varied in different experiments from 170 to 350 mm. Diamonds were mounted on seats made of B4C or cBN allowing us to collect the complete Debye rings to 0.95 Å. The collected images were integrated using the Fit2D program in order to obtain a conventional diffraction spectrum. Pressure was determined from -Fe equation or NaCl equation of state2. All loadings of DAC were made in inert atmosphere (Ar or He). To study the tiny specimens recovered from diamond anvil experiments with the transmission electron microscope (TEM) it was necessary to thin them to electron transparency. The specimen foils were thin enough to be mounted directly onto conventional Cu grids. They were subjected to Ar ion bombardment in a GATAN DUOMILL milling machine (4.5 kV, 0.5 mA). To observe and characterise the microstructures and structural state of ion-milled specimens, we used a PHILIPS CM20 FEG analytical transmission electron microscope (ATEM), operating at 200 kV. Electron diffraction experiments on the polycrystalline, fine-grained specimens were performed in selected area mode and generally yielded ring patterns; they were scanned and evaluated in the same manner as X-ray diffraction patterns. To measure the compositional variations, we employed a ThermoNoran Vantage energydispersive X-ray (EDX) spectrometer, which was attached to the ATEM. The quantification of EDX spectra was done according to the Cliff-Lorimer thin film technique4. Ab initio electronic structure and total energy calculations were carried out in the framework of the density functional theory5 using the generalized gradient approximation for the exchange-correlation energy and one-electron potential. The basis set of the so-called EMTO (exact muffin-tin orbitals) was employed, and the complete technique is described in ref. 6. The all-electron calculations were carried out. The 3p, 3d, and 4s electrons of Fe, as well as 3s and 3p electrons of Si were treated as valence electrons. The core states were recalculated at each iteration within the soft-core approximation. A sufficiently dense mesh was used for calculating reciprocal space and energy integrals, so that the total energy was converged to within 0.1 meV. Dependences of the total energy on the volume for each system were fitted using the Birch-Murnaghan equation, and theoretical values of pressure were calculated from the fit. Fig. S1 (Supplementary materials) Results of ab initio electronic structure and total energy calculations for Fe-Si alloys at different compressions (see Methods). a. Theoretically determined mixing enthalpies (in kJ/mole) of random hcp Fe0.9Si0.1 alloy (filled circles) and formation enthalpies (in kJ/mole) of B2 FeSi compound (filled squares) at different pressures. b. Calculated density of states (DOS, per unit cell) of the B2 FeSi as a function of energy E (in eV) at pressure 160 GPa. For the mixing enthalpies, the hcp phase of Fe and the fcc phase of Si were chosen as standard states at all pressures. Mixing enthalpies were also calculated for less concentrated random hcp alloys, as well as for bcc random alloys (not shown in the figure). The latter were found to transform into the former at pressures close to the bcc-hcp transition pressure in the pure Fe. So-called ground state lines connect pure Fe and points corresponding to the formation enthalpies of the B2 compound. The fact that the mixing enthalpy of a random alloy at a given pressure is above the corresponding ground state line indicates its instability with respect to the decomposition into the Si poor hcp alloy and B2 FeSi. From the inset in a one can clearly see that this tendency increases with increasing pressure. Note that at ambient pressure the phase diagram of Fe-Si system is complicated with different intermediate phases present. They were not considered in the present calculations because earlier theoretical study for this system7 predicted the B20 to B2 structural transformation in FeSi at relatively low pressure, and indicated that the latter should be the most stable high-pressure phase for the compound with equatomic composition. The Fe3Si phase in the DO3 structure is stabilized by the ferromagnetic order7, and therefore it is not supposed to be stable at high pressure and temperature due to a suppression of magnetism. A finite DOS at the Fermi level EF in b indicates that the compound should be an electric conductor. References 1. Dubrovinsky, L. et al. Chemical interaction of iron and corundum as a source of heterogeneity at the core-mantle boundary. Nature 412, 527–529 (2001). 2. Dubrovinsky, L. S., Saxena, S. K., Tutti, F. & Le Bihan, T. X-ray study of thermal expansion and phase transition of iron at multimegabar pressure. Phys. Rev. Lett. 84, 1720–1723 (2000). 3. Prokopenko, V. B., Dubrovinsky, L. S., Dmitriev, V. & Weber, H.-P. Raman spectroscopy and X-ray diffraction in situ characterization of phase transitions in cristobalite under high pressure. J. Alloys Compounds 327, 87–95 (2001). 4. Cliff, G. & Lorimer, G. W. The quantitative analysis of thin samples. J. Microsc. 103, 203 (1975). 5. Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 136, 864–871 (1964). 6. Vitos, L., Abrikosov, I. A. & Johansson, B. Anisotropic lattice distortions in random alloys from first-principles theory. Phys. Rev. Lett. 87, 156401 (2001). 7. Moroni, E. G., Wolf, W., Hafner, J. & Podloucky, R. Cohesive, structural, and electronic properties of Fe-Si compounds. Phys. Rev. B 59, 12860–12871 (1999). Fig. S1a (Supplementary Materials) Fig. S1b (Supplementary Materials)