Lab Report
advertisement

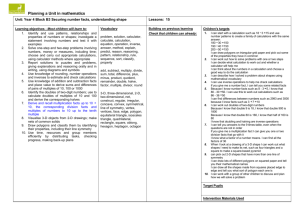
CHEM 1030L — Measurements I Name: ______________ Grade: ______________ (For Instructor use) Laboratory Report Date: ____________ Section: ______________ Lab Sta. #: ________ Lab Report Instructions: Enter text or data in the YELLOW fields. Copy results from the Calculations sheets into the BLUE fields. The highlights are provided to clarify where data, text, and calculation results should be entered by the student. The completed lab report should be printed for the instructor in black & white or gray scale. Purpose Instructions: Using 2-4 complete sentences written in third person with proper grammar and punctuation, briefly summarize the purpose of this laboratory exercise. Include the learning objectives and the major outcomes. Write the purpose of this Lab Exercise here… Data Collection Hint: ALWAYS include units of measurement and the correct number of sig. figs. Part I: Determination of the Density of Solid Objects Solid Sample # Hint: Place the data for each solid object its own column. Shape Color and other observations A.2. Mass of Object B.1 Density of Solid by Direct Measurement Length Width Height Diameter Calculated volume by direct measurement = Calculated density by direct measurement = B.2 Density of Solid by Volume Displacement Final graduated cylinder volume Initial graduated cylinder volume Displacement volume of object (mL) = Displacement volume of object (cm3) = Calculated density of object by displacement (g/cm3) = <–– Perform calculation in Calculations section and place resulting answer(s) here. <–– Perform calculation in Calculations section and place resulting answer(s) here. <–– Perform calculation in Calculations section and place resulting answer(s) here. <–– Perform calculation in Calculations section and place resulting answer(s) here. <–– Perform calculation in Calculations section and place resulting answer(s) here. Data Collection (continued) Hint: ALWAYS include units of measurement and the correct number of sig. figs. Part II. Determination of the Density of a Liquid Sample # Color and other observations Mass of beaker + liquid Mass of beaker Mass of liquid <–– Perform calculation in Calculations section and place resulting answer(s) here. Volume of liquid <–– From the pipette volume. Density of liquid (g/mL) <–– Perform calculation in Calculations section and place resulting answer(s) here. Insert Calculations section here Questions and Analysis INSTRUCTIONS: Using one or more complete sentences written in third person with proper grammar and punctuation, briefly respond to the following questions. Hint: Remember that points are awarded for each aspect of the question. 1. Explain why beakers are not used to accurately measure the volumes of liquids in the lab. How are beakers most commonly used as laboratory equipment? (Hint: An Internet search can be used to answer this.) Type the response here... 2. Is it possible to determine the mass of an object if only the volume and density is known? If so, explain how to do it. Type the response here... 3. Is it possible to determine the volume of an object if only the mass and density is known? If so, explain how to do it. Type the response here... 4. The volume of each solid object was determined two different ways: by (1) direct measurement and (2) volume displacement. For the first object (be sure to state the sample #!), what is the volume by direct measurement and the volume by displacement? Both measurements were performed on the same object, so the volumes must be the exactly same, right?? Then, why aren't they? Think carefully about how the two measurements were made. What factors could cause the volume measured by displacement to be different from the direct measurement method? Based on your discussion, which method of volume determination is more accurate – by direct measurement or by volume displacement? Explain your reasoning. (Hint: Imagine using these two methods to determine the volume of an irregularly shaped stone.) Based on your response, which density is the most accurate for each of the solid objects? Type the response here... 5. Identify the material used to make the first object. To do this, compare the calculated density of the object (be sure to use the density that you decided is most accurate!) to the density table found in eLearn. Which is the closest match? How close? How close is the next closest density? Also, compare the color of the object (and any other observations made) to what is known about the matched material. [Hint: Suppose that your comparison of densities leads you to suspect that your first object is aluminum, but you do not know what color aluminum is, you are allowed to use a reference book or the Internet to find more information about aluminum.] Sample ID # _______ Object Material: _________________ Type the response here... 6. Identify the material used to make the second object, as described above. Sample ID # _______ Object Material: _________________ Type the response here... Conclusion INSTRUCTIONS: Write an essay, using complete sentences, written in third person with proper grammar and punctuation, to briefly summarize (1) the purpose of this laboratory exercise, (2) the work that was performed, (3) observations made, (4) any problems encountered and how they could affect the results, and (5) the deliverables (densities of two solid objects and a liquid sample). Use paragraphs to separate major ideas, however, do not number the paragraphs. Type the Conclusion here...