Density Lab - TeacherWeb
advertisement

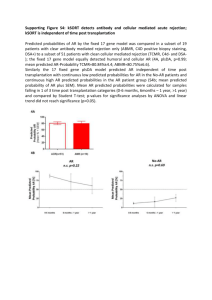
Mass, Volume, and Density Purpose: The purpose of this lab is to determine the densities of unknown materials and to use measured density values to identify unknown compounds. Background: An old riddle asks “Which is heavier, a pound of feathers or a pound of lead?” The question is nonsensical, of course, since a pound of feathers and a pound of lead both weigh the same, one pound. Nevertheless, there is clearly something different about a small lead brick and a large bag of feathers, even though they weigh the same. The key to answering the riddle is the understanding of the relationship that exists between a substance’s mass and the volume it occupies. The relationship is expressed by the physical property called density. Density is defined as the ratio of the substance’s mass to the volume it occupies. Density Mass of the the substance (g) Volume of the substance (mL) In this experiment, you will measure the mass and volume of several unknown materials. You will then use your data to explore the relationship between mass and volume of the materials and to calculate their density. Materials: Digital scale ruler and caliper 25-mL, 100-mL and 250-mL graduated cylinders Various square, rectangular, cylindrical and spherical samples Procedure: 1. Measure the mass of several separate samples of the same material to the nearest 0.01 gram, using a digital scale. Be certain that the scale has been zeroed (“tare”) before each measurement. Repeat this for all the pieces of the other remaining materials. Record the data with the proper number of significant figures. You should have a minimum of nine measurements. 2. Find the corresponding volume for each of the samples. Determine the volume by two separate methods, first by direct measurement using the measuring tools provided, and second by water displacement. Water displacement is accomplished by measuring the change in water volume in a graduated cylinder after the sample is completely submerged in the water. Record measurements to the appropriate decimal place for each method. CAUTION: DO NOT DROP THE SAMPLE IN THE GLASS CYLINDER AS IT WILL BREAK OUT THE BOTTOM OF THE CYLINDER. 3. Calculate the density for each measurement, using volumes determined by both direct measurement and water displacement. Round calculated values to the hundredth place. 4. Graph the volume versus mass for each of the different materials. Draw a line which best represents the model of the density (“best-fit” line). Calculate the equation of this line (y = mx + b) and determine the density from the slope of the curve. Identify in your data table which measurements were used to calculate Volume 1. (4 graphs per page) 5. Calculate the percent error for the calculated density of each object relative to the density predicted by the slope of a chosen graph. Record these values in the last column of your data table. Identify whether it is “Density 1 % Error” or “Density 2 % Error” in your data table. % error = l predicted density - calculated density l x 100% predicted density 6. There will be a list of materials on the board with associated actual densities to determine the identity of your unknown materials. Once you have identified your unknowns, calculate the percent error for the predicted density of each material relative to its actual density value. Indicate which predicted density you choose for each material by highlighting the equation on the chosen graph. Show all four % error calculations under the data table in your lab report. % error = l actual density - predicted density l x 100% actual density Sample Data Table: Material/S hape Dimensions (ruler) Dimensions (caliper) Volume 1 (calculated) Volume 2 (water displacement) Mass (g) Density 1 (g/mL) Density 2 (g/mL) material A (cylinder) material A (small cube) material A (large cube) material B (small cube) etc Post Lab Questions: 1. Which volume measurement method (calculated or water displacement) gave the best result for density and why? Use your data (% errors) to support your answer. 2. In the equation of the line calculated from your graph, the slope “m” is the density. What does the “b” represent in the equation? (hint: look at the units) 3. What does the “percent error” for the calculated density of each object compared to predicted density tell you about your data? What does the percent error for predicted density compared to actual density tell you? How might you account for any differences? 4. Looking at your density values, what does this experiment demonstrate about the density of a metal? What does it demonstrate about the densities of different materials? % error