Lab 1 Measurment in chemistry
advertisement

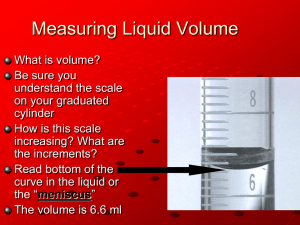
Lab 1: Measurements in Chemistry Introduction Most chemistry lab activities involve the use of various measuring instruments. The three variables you will measure most are mass, volume and temperature. Failure to obtain a satisfactory result in a lab is usually the result of improper or inaccurate use of measuring instruments. During this laboratory assignment, you will become familiar with the measuring instruments most often used in chemistry. Equipment: Materials: Triple Beam Balance Metal sample Bunsen burner Tap Water 100 mL graduated cylinder Distilled water 10 mL graduated cylinder Weighing Paper 400 mL beaker Aspirin 250 mL beaker Tums 150 mL beaker 50 mL beaker Wire gauze Safety: You will be working with an open flame, so you must wear safety Stirring rod goggles. (plus, it looks cool). Thermometer Hot Plate Crucible Tongs 1. Graduated Cylinders - Volume a. Obtain two graduated cylinders: 100 mL and 10 mL. Diagram each cylinder with each diagram clearly showing the volumetric increments on each. What is the smallest volume increment marked on each cylinder? b. Half fill each cylinder with water. Notice the shape of the surface of the water. To your sketches above, draw the shape of the water surface. This is called the meniscus. Always read the level of the liquid at the bottom of the meniscus. c. Write down the measurement of the liquid you have placed in each cylinder. Remember to estimate the last digit (if cylinder reads to nearest 1 mL, estimate tenths place, if e reads to nearest tenths place, estimate nearest hundredths place). 2. Triple Beam Balance - Mass a. Place three items (pen, coin etc.) one at a time on the balance and record the mass of each. Estimate to nearest hundredths place. (0.01g) b. Weigh one aspirin tablet. Record your data in the data table (estimate to nearest 0.01g). Read the label on the aspirin bottle and record the mass of active ingredients (aspirin) in each tablet. c. Weigh one Tums tablet. Record your data in the data table (estimate to nearest 0.01g). Read the label on the Tums bottle and record the mass of the active ingredients (calcium carbonate) in each tablet. d. What is the mass of the aspirin tablet in mg? Show your work on your lab form. e. What is the mass of the Tums tablet in mg? Show your work on your lab form. f. Calculate the percentage (by mass) of active ingredient in each of the tablets. Show your work on your lab form. 3. Beaker - Volume a. Obtain a 400 mL, 250 mL and 150 mL. How far do the markings on each instrument go? b. Place 100 mL of water in the 400 mL beaker using the markings on the beaker as your guide. Pour the water into a 100 mL graduated cylinder (if the water appears to be more than 100 mL, stop at 100 mL, pour out the water and determine the remaining amount of water with the graduated cylinder. Add additional volume to 100 mL to obtain total volume). Record the volume. Repeat this with the 250 mL and 150 mL beakers. c. Calculate the percent error for each of the beakers. Check in Chapter 3 for the formula for percent error. Assume the volume in the graduated cylinder is the accepted volume. d. What does the percent error tell you about the accuracy of the markings on a beaker? 4. Density a. Density is an easily measured physical property of a substance, often used for identifying materials. Density = mass (in grams) = m Volume (in mL) v b. Example: A section of a femur bone has a mass of 25.968 g and occupies a volume of 13.5 mL. The density of the bone fragment would be calculated as follows: D = m = 25.968 g = 1.92 g v 13.5 mL mL c. Why are there 3 significant figures in the final answer? 4b. Density of Water d. Determine the mass of a clean, dry 10 mL graduated cylinder and record its mass on your lab report (estimate to nearest 0.01g). e. ONE PARTNER will pour about 10 mL of distilled water into your 50 mL beaker and take back to your work station. f. Fill the graduated cylinder to between 4 and 5 mL of distilled water. Record the volume to nearest 0.1 mL. This means that your volume measurement will have a digit in the tenths place. Determine the mass of the cylinder and water. Record your answer with the correct units. g. Add more water and have THE OTHER PARTNER repeat step g. h. Calculate the density of water from each set of data, taking care to properly account for units and sig figs. Density results should agree fairly well. 4c. Density of a solid object i. obtain a solid metal object. Record the type of metal in the table below. Determine the mass of the sample and record j. To determine the volume, fill a graduated cylinder to a known volume and record in your data table to the nearest 0.1 mL. k. Tip the graduated cylinder sideways and slowly and carefully slip the solid into the graduated cylinder. Read and record the new volume level to the nearest 0.1 mL. The difference in the volume is the volume of the metal object. l. EACH PARTNER should select a different metal object made of different metal. Check each others work as you do your measurements. Record all results. 5. The Bunsen Burner a. Put on your safety goggles b. Light the Bunsen burner according to the instructions given by your teacher. Open and close the air window gradually and note how the shape of the flame changes. Why does this happen? Sketch a diagram of each type of flame. c. Test the temperature in the different zones of a hot flame (air window open) by holding a wire gauze (one without the white center) horizontally with a crucible tongs about 1 cm above the burner (see diagram). Note color and appearance of gauze. Now move it up through the flame until it no longer glows. d. With the air window still open, position the wire gauze vertically in the flame. This shows a vertical profile of the temperature regions of the flame. Sketch a profile of the flame and label the “cool” and “hot” regions. e. Close the air window and repeat steps c & d. 6. Thermometer - Temperature a. Record all temperatures to the nearest 0.1 ºC. This means that you will estimate your measurements to the tenths place (0.1 ºC). b. Fill your 250 mL beaker full of tap water from the sink. Place your thermometer in the beaker and once the temperature stabilizes, record the temperature. c. Fill a 150 mL beaker half-full of distilled water. Place it on a hot plate. Heat the water to boiling and record the temperature of the boiling water. Record the temperature on your report sheet. d. While waiting for the water to boil, fill your largest beaker almost to the top with ice and add distilled water. Stir gently with the stirring rod for a while, and record the lowest temperature obtained (with thermometer bulb in middle of the mixture) e. Together with your partner, get the mass of approximately 10 grams of sodium chloride. To do this, obtain the mass of the weighing paper and record the mass. Then add about 10 g of sodium chloride and record the mass again. The difference in the two masses is the mass of the sodium chloride. This procedure is called “weighing by difference”. Don’t forget your units! f. Now add the sodium chloride to the ice-water mixture, stir gently with a stirring rod for several minutes until the sodium chloride dissolves, then read and record the lowest temperature observed. My Name: Date: Hour: Partner’s Name: Measurement in Chemistry Report sheet Pre-lab questions 1. What is the purpose of this lab? 2. What is the difference between weight and mass? Which will we be measuring in lab activities? 3. A student recorded the temperature of boiling water as 100 oC. Her partner recorded the same measurement as 100.0 oC. Which is the correct way of recording the measurement? How many significant figures does each of these measurements have? 4. Why is the precision of the laboratory balance that you use in this investigation important? What effect would a less precise balance have on your results? Data 1. Graduated Cylinder a. Diagrams: Smallest volume increment: 100 mL ____________________ 10 mL _____________ c. Measurement of each liquid volume: 100 mL ____________ 10 mL ____________ 2. Triple Beam Balance - mass a. Item Mass (g) 1 2 3 b. & c. Tablet Mass of tablet (g) Mass of active ingredient (mg) Aspirin Tums d. e. f. [ Percent = mass of active ingredient x 100 ] total mass of tablet 3. Beaker - volume a. How far do the markings on each instrument go: 400 mL _____ 250 mL_____ 150 mL ____ b. The actual volume of 100 mL of water in: 400 mL ______ 250 mL _______ 150 mL _______ c. Percent error for each beaker: 400 mL _________ 250 mL ________ 150 mL __________ d. How accurate are the markings on the beaker? 4. Density c. Density of Water Data Table Trial 1 Trial 2 Mass of graduated cylinder + water Mass of empty cylinder Mass of water Volume Density of water*** ***Show the setup for both calculations below. Include all units and remember to use correct number of sig figs. BOX in your final answers!! Density of metal samples Data Table Metal 1 Metal 2 Type of metal Mass of metal sample Volume of water + metal Volume of water Mass of metal sample Volume of metal object Density of metal object*** ***Show the setup for both calculations below. Include all units and remember to use correct number of sig figs. BOX in your final answers!! 5. The Bunsen Burner b. Why does this happen to the flame? Sketch a diagram of each type. (Label each colored area.) d. Diagram: e. Diagram: 6. Thermometer Temp (ºC) Tap water Boiling water Ice water Ice water + salt Mass of weighing paper + sodium chloride Mass of weighing paper Mass of sodium chloride Conclusion: 1. If you were asked to measure exactly 100.0 mL of water what instrument would you use? 2. If you were asked to measure about 200 mL of water what instrument would you use? 3. Why should you use the same balance for an entire lab activity? 4. Why should chemicals never be weighed directly on the pan of the balance? 5. Explain, step by step, how 5.0 g of a dry chemical should be massed on a balance. 6. Explain, step by step, how 5.0 g of a liquid chemical solution should be massed on a balance.