Khalid Mirza- Oral Proposal (2)

Synthesis of Phenol Benzoquinone derivatives from New Zealand
Brown Alga Perithalia capillaris
A proposal submitted to the Faculty
Of
Drexel University
By
Khalid Baig Mirza
In partial fulfillment of the requirements for the degree of
Doctor of Philosophy
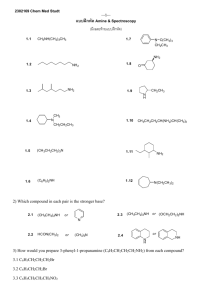
1.
Introduction
A bis-prenylated benzoquinone isolated from the New Zealand brown alga Perithalia capillaris was recently reported
1
by Perry, Sansom, Larsen, et.al. In vitro studies indicated that this compound inhibited the superoxide production by human neutrophils
(IC
50
2.1
μ M). It was also highly potent at inhibiting the proliferation of HL60 cells (IC
50
0.34
μ
M). Six other prenylated quinones and phenols along with a thaizone derivative were isolated.
Commonly used non steroidal anti-inflammatory drugs (NSAIDS) target cyclooxygenase and phospholipase enzymes in the cascade of reactions leading to an inflammation.
Certain diseases including gouty arthritis and non-atopic asthma are a result of
Superoxide production by neutrophils 2 . Superoxide inhibition has therefore been a main area of research.
HL60 cell line once isolated from a patient with acute myeloid leukemia resembles promyleocytes. These cells can be made to differentiate terminally in vitro to granulocyte-like and monocyte / macrophage-like cells. The interesting fact about these cells is that the c-myc proto-oncogene and c-myc mRNA levels decline rapidly once differentiation is induced.
3
This work intends to propose a short and viable synthesis for each of the eight natural products reportedly isolated from Perithalia capillaris
4
.
The structures of these compounds are as listed below;
1 Sansom, Larsen, Perry et.al J. Nat. Prod., 70 (12); dx.doi.org/10.1021/np070436t
2 Brent R. Copp et.al J. Nat. Prod. 2007, 70, 936-940; dx.doi.org/10.1021/ol0499699
3 Br J Cancer Suppl. 1988 Dec; 9:41-5.
4 Sansom, Larsen, Perry et.al J. Nat. Prod., 70 (12); dx.doi.org/10.1021/np070436t
2
3
Compound # 1
OH
OH
O
2
N
+
2-Acetamidophenol
Br
Prenyl Bromide
Na, Ether Reflux
O
2
N
OH
1.Aqueous NaOH
2.
Br
O
2
N
OH
Heat Strongly
Claisen [3,3] Sigmatropic Shift
O
2
N
O
1.Fe / HCl
2.NaNO
2
/ H
+
3.H
3
PO
2
OH
5 Name reactions and Reagents in Organic Synthesis, Bradford, et.al. 2005, 156-157
6 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
7 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
5
,
6
,
7
4
Compound #2
OH
OH
+
Br
Prenyl Bromide
Na, ether, reflux
Br
OH
AlCl
3
/ HCl or FeCl
3
/ HCl
OH
89
8 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
9 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
5
Compound 3
H
2
C
H
3
C
CH
3
H
2
C CH
3
SO
2
Cl
2
OH
+
S
CH
3
CH
3
Step 2
NaOH /H
2
O
CH
2
Cl
2
H
3
C
H
2
N
H
2
N
Cl
CH
3
S
+
Cl
H
2
C
CH
3
O
CH
3
CH
3
H
2
C CH
3
O
S
+
Cl
-
CH
3
CH
3
Et
3
N
H
2
C CH
3
O
C
-
H
S
+
CH
3
CH
3
H
3
C
CH
3
S CH
2
CH
3
CH
3
1. BF
3
.OEt
2
2. Raney Ni
3. NaIO
4
4. Heat
OH
CH
3
3a
O
+
AlCl
3
/ H
+
Br CH
3
CH
3
H
3
C
CH
3
S
CH
2
H
2
C CH
3
H
3
C OH
CH
3
CH
3
Small amount
O CH
3
10 , 11
Compound 3a may also be prepared by a simple condensation reaction of hydroquinone in THF at 65-70 C with isoprene in heptane; the reaction is catalyzed by Amberlyst-15 resin
12
. 3a may then be subjected to Friedel Crafts alkylation using prenylbromide and a lewis acid to obtain compound 3.
10
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (7), 1525-9; 1987
11 Sato, Miyamoto, et.al; J. Org. Chem.; 1987; 52 (24) pp 5495-5497; dx.doi.org/ 10.1021/jo00233a047
12 Banerji, Jain and Kalena; Molecules 1997 , 2 , 100–105
6
Compound # 4
O
O
O
2
N
OH
Cl
+
Br
Prenyl Bromide
1. Aq. NaOH
Heat
O
2
N
OH
Cl
CH
2
N
2
Or Aq. NaOH / CH
3
Cl
O
2
N
OCH
3
Cl
1. NaOH / Heat
2. H20 / H
+
OCH
3
O
2
N
OH
O
1.a.Sn/HCl, b. HONO, c.H
3
PO
2
CAN , CH
3
CN - H
2
O
2.
Br
OH
4a
OCH
3
O
+
The other isomer...maybe in small amount due to steric hinderance
Na , Ether Reflux
13
,
14
,
15
Compound 4a after removal of the nitro group can be converted to the product by a [1,3] sigmatropic rearrangement catalyzed by Florisil
16
13 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
14 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
15
Chung and Kim J. Am. Chem. Soc. 2001, 123, 11071-11072 dx.doi.org/10.1021/ja0115114
16 Talams, Smith, et.al; Tett. Lett.1997, 38, 4725 dx.doi.org/10.1016/S0040-4039(97)00949-0
7
Compound 5 H O
H
2
C
CH
3
CH
3
OH H
3
C
S
+ H
3
C
NHAc
NaOH /H
2
O
CH
2
Cl
2
Cl
H
2
C
H
3
C
Step 2 CH
3
Cl
H
2
N
S
CH
3
H
2
N
H
2
C
CH
3
O
SO
2
Cl
2
CH
3
CH
3
AcHN
AcHN
H
2
C CH
3
O
S
+
CH
3
Cl
-
CH
3
Et
3
N
H
2
C CH
3
O
C
-
H
S
+
CH
3
CH
3
AcHN
O
1. FC Alkylation
AlCl
3
/ H
+
Br
CH
3
CH
3
CH
3
H
3
C
AcHN
1. BF
3
.OEt
2
2. NaIO
4
3. Heat cyclization followed by Oxidative desulfurization
CH
3
S
OH
+
2. NHAc Deprotection OH
CH
3
-
/ H
2
O
3a.HONO; b. Cu
2
O /Cu
2+
; H
2
O
H
2
N
H
3
C
CH
3
S
CH
2
CH
3
OH
Small amount
CH
2
CH
3
H O
H
3
C
O
CH
3
CH
3
CH
3
CH
2
17
,
18
,
19
17 Sato, Miyamoto, et.al; J. Org. Chem.; 1987; 52 (24) pp 5495-5497; dx.doi.org/ 10.1021/jo00233a047
18 Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (7), 1525-9; 1987
19 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
8
Compound # 6
OH H
3
C
S
+ H
3
C
NHAc
NaOH /H
2
O
CH
2
Cl
2 Cl
H
2
C
H
3
C
Step 2 CH
3
Cl
H
2
N
S
CH
3
H
2
N
H
2
C
CH
3
SO
2
Cl
2
AcHN
AcHN
H
2
C CH
3
O
S
+
Cl
-
CH
3
CH
3
Et
3
N
H
2
C CH
3
O
C
-
H
S
+
CH
3
CH
3
AcHN
O
1. FC Alkylation
AlCl
3
/ H
+
H
3
C Cl
CH
3
CH
3
H
3
C
AcHN
1. BF
3
.OEt
2
2. NaIO
4
3. Heat cyclization followed by Oxidative desulfurization
CH
3
S
OH
+
2. NHAc Deprotection OH
-
/ H
2
O
3a.HONO; b. Cu
2
O /Cu
2+
; H
2
O
H
2
N
CH
2
CH
3
H
3
C
CH
3
S
OH
Small amount
CH
2
CH
3
H O
H
3
C
20
,
21
,
22
O
CH
3
CH
3
20 Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (7), 1525-9; 1987
21 Sato, Miyamoto, et.al; J. Org. Chem.; 1987; 52 (24) pp 5495-5497; dx.doi.org/ 10.1021/jo00233a047
22 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
9
OCH
3
OCH
3
NH
2
+
Compound # 7
O
S
O
O
N
H
O
N
H
CO
2
H
O
Cl
MeCl
2
OCH
3
OCH
3
NH O
AlCl
3
/ Chlorobenzene
120
OCH
3
N
OCH
3
H
O
POCl
3
, CuCN, DMF, NaBH
4
2. H
+
/H
2
O
3. SOCl
2
, EtOH
4. AcCl, Et
3
N / CH
2
Cl
2
5.CAN /CH
3
CN, H
2
O
O
S
O
O
N
H
H
N
+
O
O
N
H
CO
2
H
S
O O O
N
H
CO
2
H
HO
2
S
NH
2
C
2
H
5
OH / H
2
O / H
+
O
O
N CO
2
Et
O
23
,
24
,
25
23 Tzeng, Lee, Chen, Wang Tetrahedron Letters Volume 37, Issue 35, 26 August 1996, Pages 6369-6370; dx.doi.org/ 10.1016/0040-4039(96)01364-0
24 Copp, Brent, et.al. J. Nat. Prod. 2007, 70, 936-940; dx.doi.org/ 10.1021/np060626o
25 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
10
Compound # 8
OH
OH
OH OH
1. Sn / HCl, OH
-
2. HONO
OH
Na , Ether Reflux
+
Br
3. Cu
2
O, Cu
+2
, H
2
O
26
NO
2
Prenyl Bromide
NO
2
OH
The compounds are extracted from methylene chloride and water, dried over anhydrous
MgSO4 or anhydrous CaCl2, further dried in a vacuum dessicator. The compounds are analyzed using various NMR techniques. The results are compared to the published data for a confirmation of the structural identity of the compounds.
REFERENCES:
Sansom, Larsen, Perry et.al J. Nat. Prod., 70 (12); dx.doi.org/10.1021/np070436t
2 Brent R.Copp et.al J. Nat. Prod. 2007, 70, 936-940; dx.doi.org/10.1021/ol0499699
3 Br J Cancer Suppl. 1988 Dec; 9:41-5.
4 Sansom, Larsen, Perry et.al J. Nat. Prod., 70 (12); dx.doi.org/10.1021/np070436t
5 Name reactions and Reagents in Organic Synthesis, Bradford, et.al. 2005, 156-157
6 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
7 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
8 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
9 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
10 Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (7), 1525-9; 1987
11 Sato, Miyamoto, et.al; J. Org. Chem.; 1987; 52 (24) pp 5495-5497; dx.doi.org/ 10.1021/jo00233a047
12 Banerji, Jain and Kalena; Molecules 1997 , 2 , 100–105
13 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
14 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
15
Chung and Kim J. Am. Chem. Soc. 2001, 123, 11071-11072 dx.doi.org/10.1021/ja0115114
16 Talams, Smith, et.al; Tett. Lett.1997, 38, 4725 dx.doi.org/10.1016/S0040-4039(97)00949-0
17 Sato, Miyamoto, et.al; J. Org. Chem.; 1987; 52 (24) pp 5495-5497; dx.doi.org/ 10.1021/jo00233a047
18 Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (7), 1525-9; 1987
19 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
20 Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (7), 1525-9; 1987
21 Sato, Miyamoto, et.al; J. Org. Chem.; 1987; 52 (24) pp 5495-5497; dx.doi.org/ 10.1021/jo00233a047
22 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
23 Tzeng, Lee, Chen, Wang Tetrahedron Letters Volume 37, Issue 35, 26 August 1996, Pages 6369-6370; dx.doi.org/ 10.1016/0040-4039(96)01364-0
24 Copp, Brent, et.al. J. Nat. Prod. 2007, 70, 936-940; dx.doi.org/ 10.1021/np060626o
26 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
11
25 Mehta and Pan; Org. Lett. 2004 Vol 6, No. 5 811-813 dx.doi.org/10.1021/ol0499699
26 Solomons, Fryhle; Organic Chemistry; 9/e 2007; John Wiley & Sons, Inc.
12