General Aspects of Spectroscopy
advertisement
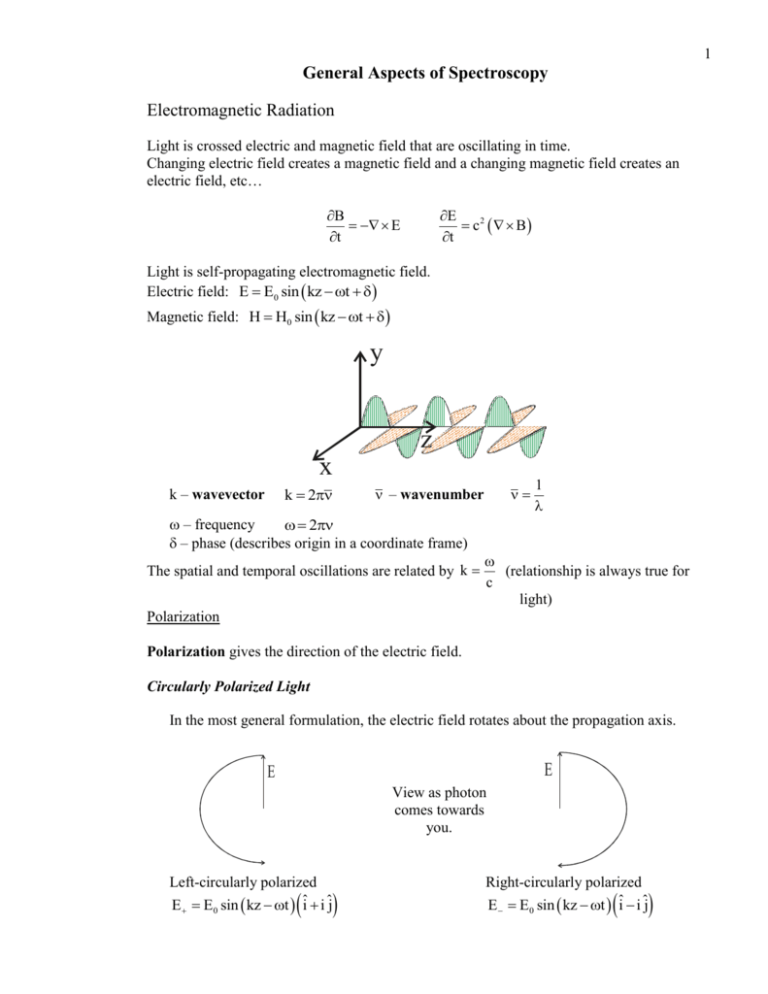
1 General Aspects of Spectroscopy Electromagnetic Radiation Light is crossed electric and magnetic field that are oscillating in time. Changing electric field creates a magnetic field and a changing magnetic field creates an electric field, etc… B E t E c2 B t Light is self-propagating electromagnetic field. Electric field: E E0 sin kz t Magnetic field: H H0 sin kz t y z x k 2 k – wavevector – wavenumber – frequency 2 – phase (describes origin in a coordinate frame) The spatial and temporal oscillations are related by k 1 (relationship is always true for c light) Polarization Polarization gives the direction of the electric field. Circularly Polarized Light In the most general formulation, the electric field rotates about the propagation axis. E E View as photon comes towards you. Left-circularly polarized E E0 sin kz t ˆi i ˆj Right-circularly polarized E E0 sin kz t ˆi i ˆj 2 Photon has one unit of angular momentum. L - two polarizations two values for ML: ML = 1 - note ML = 0 is missing (no longitudinal field) Plane Polarized Light Plane polarized light is a linear combination of circularly polarized photons. Consider “animation” below Ey E+ E- E+ Ey E- E+ Ey= 0 E- E + EEy E+ EEy Because it is often simplest to consider molecules in a Cartesian coordinate system planepolarized light is often very useful to probe the property of matter. Time-dependent Schrödinger Equation So far in our study of quantum chemistry, we have been studying Hamiltonians and solutions to the Schrödinger equation that have been time-independent (that is, operators and wavefunctions do not change with time. The interaction of light with matter is time-dependent process, so let us briefly consider the time-dependent Schrödinger equation. H i t Thus, two operators exist for the energy. Ê H , Ê i t The time-dependent wavefunctions are related to the time-independent wavefunctions via the following solution. n r, t n r e i En t 3 Time-dependent Perturbation Theory Background In searching for solutions to the time-dependent Schrödinger equation, the Hamiltonian is split into a time-independent portion and time-dependent portion. H(t) H0 H1 t Perturbation theory is now applied where the time-dependent portion of the Hamiltonian is considered the perturbation. The total wavefunction is constructed from a linear combination of zeroth-order wavefunctions, just as in time-independent perturbation theory; however, the coefficients are allowed to be time dependent. a1 t 1 t a 2 t 2 t a 3 t 3 t Two-state wavefunction Generally, a time-dependent interaction involves only two states so that the total wavefunction is a linear combination of a lower energy state and a higher energy state. a1 t 1 t a 2 t 2 t The remainder of the perturbation analysis is similar to the analysis for first-order timeindependent perturbation theory and it yields expressions for the time-dependent coefficients. da1 t a 2 t 1 H12 t ei0 t dt i da 2 t a1 t 1 H 21 t ei0 t dt i E E 1 where H12 t 1 r H1 t 2 r d and 0 2 1 Aside: H12 is called a matrix element. The matrix element is analogous to an expectation value. A matrix element describes the probability of a system changing from state 1 to state 2 under the influence of the 1 perturbation, H t . (Perturbation does not have to depend upon time.) 1 The fact that the coefficients depend on each other reflects the idea that the while the perturbation is applied, the system oscillates between the two states. 4 Solution for wavefunction coefficients To find the a2 coefficient, solve the above differential equation and allow that the interaction is very small so that a1 remains approximately constant at a value of 1. t 1 1 If a2 is initially zero (i.e., a2(0) = 0), then a 2 t H12 t ei0 t dt i 0 Let H t 2H cos t H eit e it 1 1 1 Note the difference between 0 (a difference in energy levels of the quantum state) and (the frequency of the perturbation (light)) The probability that the system is in state 2 is the square of the a2 coefficient. 4H12 H 21 sin 2 1 2 0 t 1 P2 a 2 2 1 2 0 2 Fermi’s Golden Rule Fermi’s Golden Rule relates how quickly a transition occurs between a lower energy level (1) and a higher energy level (2). The transition rate, W21 , is the time derivative of the probability of the state being in the higher energy level. W21 dP2 dt Starting with the probability found in the previous section and after some sophisticated calculus and a few reasonable physical assumptions, Fermi’s Golden Rule can be stated as dP2 2 1 2 H12 N E 2 dt is density of energy level near energy level 2. W21 where N E2 Key points of Fermi’s Golden Rule 1. Transition rate depends on the square of the transition matrix element. - If spectroscopic transition, matrix element is transition dipole matrix element. 2. Transition rate is independent of time. 3. The higher the density of energy levels (more levels within a smaller energy range), the higher the transition rate. 5 Stimulated and Spontaneous Interactions of Dipole Radiation Absorption For an interaction between light and matter to occur (absorption), the oscillating dipole electric field of the incident light must be able to induce a dipole within the matter. Absorption is always stimulated. Absorption is proportional to the power of incident light. Wf i dN number of molecules dt absorbed per unit time W NB N – number of molecules B – Einstein absorption coefficient - energy density 8kT 4 Emission Light is emitted only when dipole in matter oscillates. Such an oscillation can be spontaneous or it can be stimulated with a photon. Stimulated emission is proportional to the power of the incident light. Wfi dN number of molecules dt emitting per unit time Wfi NB B′ – Einstein stimulated emission coefficient Spontaneous emission is not dependent on the power of the incident light. Wfi NA B′ – Einstein spontaneous emission coefficient The total emission rate is the sum of the stimulated and spontaneous rates. Wfi N A B Spontaneous emission is “normal” emission, but spontaneous emission in more difficult to explain at a fundamental level. (Why does matter start spontaneously shaking as to create a dipole?) 6 Spectral Lineshapes Motivation: Transitions between quantized energy levels have definite energies, thus a spectrum should have infinitely small line widths. However, we find that the peaks in spectra do have finite widths. The vast majority of these line widths come from physical processes, not some sort of instrument error. Because line widths have physical explanations, measurement of line widths often yields useful information. Types of Line Broadening Doppler Broadening Molecules moving away from a photon sees the photon with a smaller frequency (longer wavelength) than if the molecule was motionless. - Phenomenon is known as a red shift. c receding cv where c is the speed of light and v is velocity of molecule Molecules moving toward a photon sees the photon with a higher frequency (smaller wavelength) than if the molecule was motionless. - Phenomenon is known as a blue shift. c approaching cv 7 Doppler broadening depends on knowing the velocities of the molecules. Thus, we need an expression that relates the velocity of molecule to a more easily measurable quantity (such as temperature). For ideal gas molecules, the distribution of molecular velocities is assumed to be the Maxwell distribution. 3 2 Mv M 2 2 2RT f v 4 v e 2RT where f(v) is the fraction of molecules in a gas sample with a velocity, v, at a given temperature. The molar mass of the gas is given as M. At relatively low temperatures, the distribution is approximately Gaussian (i.e., “bell-shaped”) Thus a spectral line width influenced by Doppler broadening has Gaussian line shape. 1 2v 2kT ln 2 2 c M Line widths are almost always measured by “Full Width at Half Height”. Thus measuring the Full Width at Half Height, i.e., the line width, yields information about the velocity of the molecule. Natural Broadening When a molecule (or atom) spends a finite amount of time in an excited state, an uncertainty in the energy of the state exists. Recall the Heisenberg uncertainty principle. E t 2 An uncertainty in the energy of the excited state yields an uncertainty in the transition frequency. An uncertainty in the transition frequency yields a distribution of transition frequency. 1 1 f lm 2 2 where is the lifetime of the excited state, lm is the transition frequency, is the frequency of the incident light and is the line width. 8 uncertainty transition transition spectrum spectrum The name of the spectral line shape is a called a Lorentzian line shape. Note: Gaussian and Lorentzian lines look similar but, 1. Gaussian lines are broader near the center of the peak 2. Lorentzian lines are broader in the tail portion of the peak. Lorentzian G aussian Pressure Broadening (Collisional Broadening) Collisions between molecules jostle the charge distribution of molecules. The jostling causes an induced dipole that can subsequently absorb or emit radiation. The collisions stimulate absorption and emission. The uncertainty in the energy is now related to the time between collisions. 2 1 1 S 2 2 lm 2 2 lm 2 2 The shape of the curve is approximately Lorentzian. For frequencies “near resonance”, that is lm , the first term dominates and the second term becomes important only when a gas sample is at high pressure. The time between collisions is related to the attraction between molecules; therefore, line width investigations are a common technique used to investigate intermolecular forces. Wall Broadening Same as pressure broadening except that now collisions with container wall are important. Generally, this effect is undesirable. The effect is minimized by keeping surface area to volume ratio low.