Solubility Product Principle: Ksp Worksheet
advertisement
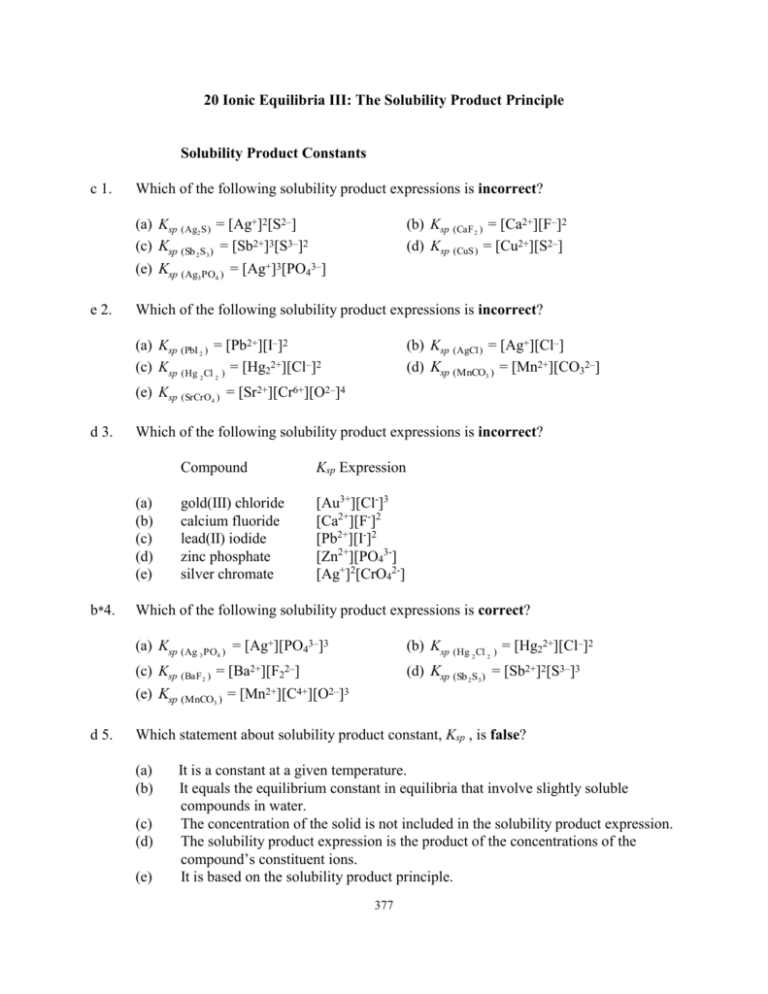
20 Ionic Equilibria III: The Solubility Product Principle Solubility Product Constants c 1. Which of the following solubility product expressions is incorrect? (a) Ksp (Ag2 S) = [Ag+]2[S2–] (c) Ksp (Sb 2S3) = [Sb2+]3[S3–]2 (b) Ksp (CaF 2 ) = [Ca2+][F–]2 (d) Ksp (CuS) = [Cu2+][S2–] (e) Ksp (Ag3 PO4 ) = [Ag+]3[PO43–] e 2. Which of the following solubility product expressions is incorrect? (a) Ksp (PbI 2 ) = [Pb2+][I–]2 (c) Ksp (Hg Cl ) = [Hg22+][Cl–]2 2 (b) Ksp (AgCl) = [Ag+][Cl–] (d) Ksp (MnCO3 ) = [Mn2+][CO32–] 2 (e) Ksp (SrCrO4 ) = [Sr2+][Cr6+][O2–]4 d 3. Which of the following solubility product expressions is incorrect? (a) (b) (c) (d) (e) b*4. Compound Ksp Expression gold(III) chloride calcium fluoride lead(II) iodide zinc phosphate silver chromate [Au3+][Cl-]3 [Ca2+][F-]2 [Pb2+][I-]2 [Zn2+][PO43-] [Ag+]2[CrO42-] Which of the following solubility product expressions is correct? (a) Ksp (Ag 3 PO4 ) = [Ag+][PO43–]3 (b) Ksp (Hg 2 Cl 2 ) = [Hg22+][Cl–]2 (d) Ksp (Sb 2S3) = [Sb2+]2[S3–]3 (c) Ksp (BaF 2 ) = [Ba2+][F22–] (e) Ksp (MnCO3 ) = [Mn2+][C4+][O2–]3 d 5. Which statement about solubility product constant, Ksp , is false? (a) (b) (c) (d) (e) It is a constant at a given temperature. It equals the equilibrium constant in equilibria that involve slightly soluble compounds in water. The concentration of the solid is not included in the solubility product expression. The solubility product expression is the product of the concentrations of the compound’s constituent ions. It is based on the solubility product principle. 377 378 Determination of Solubility Product Constants e 6. How is the Ksp of Ca3(PO4)2 related to s, the molar solubility of Ca3(PO4)2? (a) Ksp = 4s5 (d) Ksp = 54s4 b 7. (c) 5.3 x 10–12 (b) 4.9 x 10–26 (e) 1.9 x 10–33 (c) 7.1 x 10–35 At 25°C, 1.4 x 10–5 mole of Cd(OH)2 dissolves to give 1.0 liter of saturated aqueous solution. What is the solubility product for Cd(OH)2? (a) 1.7 x 10–5 (d) 5.8 x 10–15 c 10. (b) 8.1 x 10–9 (e) 6.7 x 10–11 Calculate the solubility product constant for aluminum hydroxide. Its molar solubility is 2.9 x 10–9 mole per liter at 25°C. (a) 9.8 x 10–26 (d) 2.1 x 10–34 c 9. (c) Ksp = 18s5 The molar solubility of BaCO3 is 9.0 x 10–5 M at 25°C. What is the solubility product constant for BaCO3? (a) 1.2 x 10–8 (d) 4.0 x 10–15 e 8. (b) Ksp = 27s3 (e) Ksp = 108s5 (b) 2.9 x 10–10 (e) 4.1 x 10–12 (c) 1.1 x 10–14 One liter of saturated zinc hydroxide solution contains 0.000222 g of dissolved Zn(OH)2. Calculate Ksp for Zn(OH)2. (a) 4.8 x 10–12 (d) 7.1 x 10–14 (b) 2.6 x 10–16 (e) 1.1 x 10–17 (c) 4.5 x 10–17 b 11. The solubility of Ce(OH)3 is 9.93 x 10–4 g per liter at 25°C. Calculate the solubility product for Ce(OH)3. (a) 5.2 x 10–6 (d) 4.0 x 10–14 (b) 2.0 x 10–20 (e) 1.6 x 10–18 (c) 7.3 x 10–20 d 12. The solubility of Fe(OH)2 is 3.00 x 10–3 g in 2.00 liters at 18°C. What is its Ksp at 18°C? (a) 4.92 x 10–12 (d) 1.86 x 10–14 (b) 6.03 x 10–9 (e) 2.11 x 10–11 (c) 2.44 x 10–10 Chapter 20 379 b 13. The solubility of cerium periodate, Ce(IO4)4, is 0.015 g/mL of water at 25°C. Calculate its solubility product constant. Its formula weight is 904 g/mol. (a) 8.1 x 10–14 (d) 4.1 x 10–12 c 14. (b) 3.2 x 10–7 (e) 6.9 x 10–22 The solubility of cobalt(III) hydroxide is 54 pg/100. mL of water at 25°C. Calculate the Ksp for cobalt(III) hydroxide at 25°C. (a) 5.8 x 10–46 (d) 5.8 x 10–50 e 15. (c) 9.4 x 10–24 (b) 1.7 x 10–45 (e) 1.6 x 10–48 (c) 1.6 x 10–44 Magnesium hydroxide is a slightly soluble substance. If the pH of a saturated solution of Mg(OH)2 is 10.49 at 25°C, calculate Ksp for Mg(OH)2. (a) 8.8 x 10–16 (d) 4.4 x 10–14 (b) 4.2 x 10–15 (e) 1.5 x 10–11 (c) 6.0 x 10–10 Uses of Solubility Product Constants e 16. How is the molar solubility, s, related to the Ksp for Fe(OH)3; i.e., s = ________? (a) (Ksp)1/4 a 17. (b) (Ksp /4)1/3 (c) (Ksp)1/2 (b) 1.7 x 10–5 M (e) 1.8 x 10–10 M (c) 4.2 x 10–5 M The value of Ksp for SrSO4 is 2.8 x 10–7. What is the molar solubility of SrSO4? (a) 7.6 x 10–7 M (d) 5.7 x 10–3 M c 19. (e) (Ksp /27)1/4 Calculate the molar solubility of AgCl at 25°C. Ksp = 1.8 x 10–10. (a) 1.3 x 10–5 M (d) 4.6 x 10–5 M c 18. (d) (Ksp /16)1/4 (b) 5.8 x 10–13 M (e) 1.3 x 10–8 M (c) 5.3 x 10–4 M The Ksp for magnesium arsenate is 2.1 x 10–20 at 25°C. What is the molar solubility of Mg3(AsO4)2 at 25°C? (a) 6.7 x 10–3 M (d) 7.0 x 10–5 M (b) 3.6 x 10–4 M (e) 1.4 x 10–6 M (c) 4.5 x 10–5 M b 20. Ksp for silver arsenate is 1.1 x 10–20. What is the molar solubility of Ag3AsO4? (a) 2.2 x 10–7 M (d) 4.1 x 10–7 M (b) 4.5 x 10–6 M (e) 1.0 x 10–10 M (c) 6.2 x 10–8 M 380 a 21. Calculate the molar solubility of nickel phosphate at 25°C. Its Ksp is 5.0 x 10–31. (a) 3.4 x 10–7 M (d) 7.1 x 10–16 M e 22. (c) 6.1 x 10–7 M Which of the following compounds has the lowest molar solubility in water at 25°C? As Ag4Fe(CN)6 dissolves, it dissociates as follows. Ag4[Fe(CN)6](s) 4Ag+ + [Fe(CN)6]4– (a) (b) (c) (d) (e) e 23. (b) 8.7 x 10–7 M (e) 5.0 x 10–11 M Compound Ag2CO3 Ag4Fe(CN)6 Ag3PO4 Ag2SO4 Bi(OH)3 (see above) Ksp______ 8.1 x 10–12 1.6 x 10–41 1.3 x 10–20 1.7 x 10–5 3.2 x 10–40 Calculate the concentration of OH– ions in a saturated Mn(OH)2 solution. The solubility product for Mn(OH)2 is 4.6 x 10–14. (a) 1.0 x 10–5 M (d) 3.2 x 10–5 M (b) 2.0 x 10–5 M (e) 4.5 x 10–5 M (c) 1.6 x 10–5 M e*24. Calculate the pH in a saturated Mn(OH)2 solution. The solubility product for Mn(OH)2 is 4.6 x 10–14. (a) 9.0 (b) 9.2 (c) 9.3 (d) 9.5 (e) 9.7 b 25. Calculate the concentration of F– ions in saturated CaF2 solution at 25°C. Ksp = 3.9 x 10-11. (a) 2.1 x 10–4 M (d) 0.032 M (b) 4.3 x 10–4 M (e) 0.10 M (c) 0.016 M b 26. Calculate [Al3+] in a saturated Al(OH)3 solution at 25°C. Ksp = 1.9 x 10–33 (a) 6.6 x 10–9 M (d) 8.4 x 10–18 M (b) 2.9 x 10–9 M (e) 1.5 x 10–7 M (c) 1.5 x 10–17 M b*27. Calculate the pH of a saturated aqueous solution of Co(OH)2. Ksp is 2.5 x 10–16. (a) 8.60 (b) 8.90 (c) 9.10 (d) 9.20 (e) 9.40 Chapter 20 381 b 28. What mass of Zn(OH)2 is contained in 1.0 liter of saturated solution? Ksp = 4.5 x 10–17. (a) 0.00011 g (d) 0.010 g e 29. (b) 0.00022 g (e) 0.016 g (c) 0.00044 g What mass of CaF2 is contained in 1.0 liter of saturated solution? Ksp = 3.9 x 10–11. (a) 0.0010 g (b) 0.0028 g (c) 0.0034 g (d) 0.010 g (e) 0.017 g a*30. Calculate the solubility of Mg(OH)2 in g per 100. mL of water. Ksp = 1.5 x 10–11. (a) 9.1 x 10–4 g/100. mL (d) 1.8 x 10–3 g/100. mL (b) 3.6 x 10–3 g/100. mL (e) 5.0 x 10–6 g/100. mL (c) 6.6 x 10–5 g/100. mL a*31. The solubility product constant for MgF2 is 6.4 x 10–9. How many grams of MgF2will dissolve in 150 mL of H2O at 25°C? (a) 1.1 x 10–2 g (d) 6.0 x 10–4 g (b) 5.8 x 10–3 g (e) 2.2 x 10–3 g (c) 4.7 x 10–1 g b 32. Given the following values of Ksp for four slightly soluble sulfides at 25°C. Sulfide CdS CuS Ksp_ 3.6 x 10–29 8.7 x 10–36 Sulfide PbS MnS Ksp______ 8.4 x 10–28 5.1 x 10–15 Which one of the following ions exists in the lowest concentration in a solution in which the sulfide ion concentration had been fixed at some (fairly large) constant value at 25°C? (a) Cd2+ (b) Cu2+ (c) Pb2+ (d) Mn2+ (e) Cannot be answered without knowing the sulfide ion concentration. b 33. AgCl would be least soluble at 25°C in _____. (a) pure water (d) 0.1 M HNO3 (b) 0.1 M CaCl2 (c) 0.1 M HCl (e) It is equally soluble in all of the preceding substances. d 34. Ksp for lead fluoride is 3.7 x 10–8. What is the molar solubility of PbF2 in 0.10 M NaF? (a) 4.6 x 10–5 M (d) 3.7 x 10–6 M (b) 1.9 x 10–4 M (e) 8.5 x 10–8 M (c) 3.7 x 10–7 M 382 b 35. How many moles of PbSO4 will dissolve in 1.0 liter of 0.010 M K2SO4? The solubility product for PbSO4 is 1.8 x 10–8. (a) 5.0 x 107 c 36. (b) 1.8 x 10–6 (c) 1.8 x 10–4 (d) 1.8 x 10–2 (e) 5.0 x 10–3 How many grams of AgCl will dissolve in 1.0 L of 0.25 M CaCl2? Ksp (AgCl) = 1.8 x 10–10 (a) 3.6 x 10–10 (b) 1.0 x 10–7 (c) 5.1 x 10–8 (d) 1.3 x 10–8 (e) 7.2 x 10–10 d 37. How many grams of MgF2 will dissolve in 150. mL of 0.100 M NaF solution? Ksp for MgF2 = 6.4 x 10–9 (a) 6.2 x 10–7 g (d) 6.0 x 10–6 g e 38. (b) 4.1 x 10–6 g (e) 9.3 x 10–7 g (c) 1.0 x 10–5 g If aqueous solutions of the following pairs of compounds are mixed, which combination might result in the formation of a precipitate? (a) MgCl2 + Na2S (d) CrCl3 + Ni(NO3)2 (b) NH4ClO4 + K2S (e) Na3PO4 + Cr(NO3)3 (c) BaCl2 + Ca(CH3COO)2 d 39. We mix each of the following pairs of solutions together. In which case would we not expect a precipitate (solid) to be formed? (a) KCl solution and AgNO3 solution (b) Pb(NO3)2 solution and H2SO4 solution (c) CuCl2 solution and Na2S solution (d) CaCl2 solution and HNO3 solution (e) A precipitate would be likely to form in every case. a 40. When we mix together, from separate sources, the ions of a slightly soluble ionic salt, the salt will begin to precipitate if Qsp ____ Ksp, and will continue to precipitate until Qsp _____ Ksp. (a) is greater than; equals (c) is less than; equals e 41. (b) is less than; is greater than (d) equals; is less than (e) equals; is greater than Ksp for CaF2 is 3.9 x 10–11 and Ksp for PbF2 is 3.7 x 10–8. If 200. mL each of 5.0 x 10–3 M NaF, 2.0 x 10–5 M Ca(NO3)2 , and 3.0 x 10–3 M Pb(NO3)2 solutions are mixed, (a) only CaF2 will precipitate. (b) only PbF2 will precipitate. (c) both CaF2 and PbF2 will precipitate, and the precipitate should be visible. (d) both CaF2 and PbF2 will precipitate, but the precipitate should not be visible. (e) neither CaF2 nor PbF2 will precipitate. Chapter 20 383 d 42. The Ksp for Fe(IO3)3 is 10–14. We mix two solutions, one containing Fe3+ and one containing IO3– ions at 25°C. At the instant of mixing, [Fe3+] = 10–4 M and [IO3–] = 10–5 M. Which one of the following statements is true? (a) A precipitate forms, because Qsp > Ksp. (b) A precipitate forms, because Qsp < Ksp. (c) No precipitate forms, because Qsp > Ksp. (d) No precipitate forms, because Qsp < Ksp. (e) None of the preceding statements is true. d 43. Calculate the [Ca2+] required to start the precipitation of calcium fluoride, CaF2, from a solution containing 0.0025 M F– at 25°C. Ksp for CaF2 = 3.9 x 10–11. (a) 6.4 x 10–7 M (d) 6.2 x 10–6 M (b) 5.2 x 10–10 M (e) 1.6 x 10–8 M (c) 4.8 x 10–3 M b 44. What concentration of Al3+ must be exceeded in a solution buffered at pH = 4.80 in order to initiate precipitation of Al(OH)3 at 25°C? Ksp for Al(OH)3 = 1.9 x 10–33. (a) 6.3 x 10–10 M (d) 5.3 x 10–7 M (b) 7.6 x 10–6 M (e) 2.2 x 10–8 M (c) 4.1 x 10–4 M b 45. Calculate the concentration of carbonate ion in a saturated solution of calcium carbonate to which calcium chloride has been added until [Ca2+] = 0.015 M at 25°C. Ksp for CaCO3 = 2.8 x 10–9. (a) 4.2 x 10–11 M (d) 6.3 x 10–13 M e 46. (c) 1.2 x 10–5 M If solid KOH is added to 0.10 M Mg(NO3)2 solution until the pH reaches 12.00, what concentration of Mg2+ remains in solution? Assume no volume change due to addition of solid KOH. Ksp for Mg(OH)2 is 1.5 x 10–11. (a) 3.8 x 10–8 M (d) 1.5 x 10–9 M e 47. (b) 1.9 x 10–7 M (e) 1.5 x 10–2 M (b) 1.0 x 10–2 M (e) 1.5 x 10–7 M (c) 5.0 x 10–3 M What is the minimum concentration of iodide ion required to limit the concentration of lead ion in solution to a maximum of 1.0 x 10–6 M? Ksp for PbI2 is 8.7 x 10–9. (a) 1.7 x 10–2 M (d) 8.7 x 10–3 M (b) 1.8 x 10–1 M (e) 9.3 x 10–2 M (c) 7.6 x 10–5 M 384 d 48. An aqueous solution of silver bromide is adjusted so that the concentration of silver ion in solution is limited to a maximum of 1.0 x 10–9 M. Which of the following statements about the solution is false? Ksp for AgBr is 3.3 x 10–13. (a) (b) (c) (d) (e) The minimum concentration of bromide needed for this limit is 3.3 x 10–4 M. If the concentration of bromide is 1.5 x 10-6, no precipitate will form. If the concentration of bromide is held precisely at 3.3 x 10-4, neither forward nor reverse process is favored. Adjusting the bromide concentration always effects the silver concentration. If [Br-] is raised to 1 x 10-3 M, the silver concentration drops to 3.3 x 10-10. Fractional Precipitation e 49. Which of the following responses contains all of the true statements? I. II. III. An ion may never be completely precipitated from solution, even when the precipitating reagent is used in large excess. If solid NaCl is added slowly to a saturated solution of NiS in contact with solid NiS, nothing significant will happen to the NiS. Consider two relatively insoluble 1:1 salts (i.e., one cation and one anion per formula unit) containing the same cation, whose solubility products differ by 104. More than 90% of one anion can be precipitated before the other precipitates when the common cation is added to a solution of the two anions. (a) I and III (b) I and II (c) II and III (d) III (e) I, II, and III d 50. A solution is 0.012 M in Pb(NO3)2 and 0.20 M in Sr(NO3)2. Solid Na2SO4 is added until a precipitate just begins to form. The precipitate is _________ and the concentration of sulfate ion at this point is ___________. Ksp for PbSO4 is 1.8 x 10–8 and for SrSO4 is 2.8 x 10–7. (a) SrSO4; 2.6 x 10–7 M (d) PbSO4; 1.5 x 10–6 M c 51. (b) SrSO4; 8.3 x 10–7 M (e) SrSO4; 1.4 x 10–6 M (c) PbSO4; 6.3 x 10–6 M Suppose we have a solution that is 0.10 M in Ca2+ and also 0.10 M in Pb2+ ions. We wish to separate these ions by fractional precipitation of one of them as the carbonate. We do this by adding a source of carbonate ions, such as solid Na2CO3. Use the Ksp values, 4.8 x 10–9 for CaCO3 and 1.5 x 10–13 for PbCO3. Neglect any change in volume due to addition of solid sodium carbonate. Which will begin to precipitate from solution first — CaCO3 or PbCO3 — and what will be the [CO32–] when this begins (whether or not we can see it!)? (a) CaCO3, 4.8 x 10–8 M (d) PbCO3, 1.5 x 10–13 M (b) CaCO3, 4.8 x 10–9 M (e) PbCO3, 7.2 x 10–21 M (c) PbCO3, 1.5 x 10–12 M Chapter 20 385 d 52. A solution is 0.10 M in Pb(NO3)2 and 0.10 M in AgNO3. Solid NaI is added until the second solid compound just begins to precipitate. Which compound precipitated first — PbI2 or AgI — and what was the [I–] when the second compound began to precipitate? Ksp = 8.7 x 10–9 for PbI2 Ksp = 1.5 x 10–16 for AgI (a) PbI2, 5.9 x 10–4 M (d) AgI, 2.9 x 10–4 M (b) AgI, 9.3 x 10–5 M (e) AgI, 8.7 x 10–8 M (c) PbI2, 4.4 x 10–3 M d 53. Solid Na2SO4 is added to a solution that is 0.30 M in both Sr2+ and Pb2+. Assuming no volume change, what will be the [Pb2+] at the point at which SrSO4 just begins to precipitate at 25°C? Ksp for SrSO4 = 2.8 x 10–7 and for PbSO4 = 1.8 x 10–8. (a) 0.24 M (b) 0.16 M (c) 0.30 M (d) 0.019 M (e) 0.040 M b*54. Solid sodium chloride is added to a solution that is 0.0100M in silver nitrate and 0.0100 M in lead(II) nitrate. Assuming no volume change, calculate the percent of silver that will be precipitated when the lead chloride just begins to precipitate. Ksp = 1.8 x 10-10 for AgCl Ksp = 1.7 x 10-5 for PbCl2 (a) 99.8941% (d) 95.9000% (b) 99.9996% (e) 98.9413% (c) 99.9989% d 55. The solubility product for barium chromate, BaCrO4, is 2.0 x 10–10 and the solubility product for lead chromate, PbCrO4, is 1.8 x 10–14. If solid Na2CrO4 is added slowly to a solution which is 0.010 M in Ba(NO3)2 and 0.010 M in Pb(NO3)2, what will the concentration of Pb2+ be just before BaCrO4 begins to precipitate? Assume no volume change due to the addition of solid Na2CrO4. (a) 4.0 x 10–7 M (d) 9.0 x 10–7 M e 56. (b) 2.0 x 10–8 M (e) 1.4 x 10–5 M (c) 1.8 x 10–14 M A solution is 0.0010 M in both Ag+ and Au+. Some solid NaCl is added slowly until the second solid compound just begins to precipitate. What is the concentration of Au+ ions at this point? Ksp for AgCl = 1.8 x 10–10 and for AuCl is 2.0 x 10–13. (a) 2.0 x 10–10 M (d) 3.0 x 10–4 M (b) 4.5 x 10–7 M (e) 1.1 x 10–6 M (c) 1.8 x 10–7 M d 57. If solid AgNO3 is slowly added to a solution that is 0.050 M in NaI and 0.12 M in NaCl, what is the concentration of I– when AgCl just begins to precipitate? Ksp for AgCl = 1.8 x 10–10, Ksp for AgI = 1.5 x 10–16 (a) 1.0 x 10–9 M (d) 1.0 x 10–7 M (b) 9.2 x 10–9 M (e) 6.7 x 10–7 M (c) 8.5 x 10–8 M 386 a 58. At 25°C the Ksp for MgCO3 is 4.0 x 10–5 and that for BaCO3 is 8.1 x 10–9. Assuming both magnesium ions and barium ions are present in 1.0 x 10–3 M concentrations, what is the equilibrium concentration of carbonate ions that will precipitate the maximum concentration of Ba2+ without precipitating any Mg2+ at 25°C? (a) 4.0 x 10–2 M (d) 5.8 x 10–2 M (b) 4.2 x 10–3 M (e) 3.2 x 10–2 M (c) 3.5 x 10–3 M d 59. Solid lead nitrate is added slowly to a solution that is 0.010 M in sodium sulfate and 0.010 M in sodium chromate at 25°C. What percent of the chromate ions remain in solution, i.e., unprecipitated, just before lead sulfate begins to precipitate? Ksp for PbSO4 = 1.8 x 10–8, Ksp for PbCrO4 = 1.8 x 10–14 (a) 0.18% a 60. (b) 0.018% (c) 0.0018% (d) 0.00010% (e) 0.00018% Solid silver nitrate is added slowly to a solution that is 0.0010 M in sodium chloride and 0.0010 M in sodium bromide. What % of the bromide ions remain in solution, i.e., unprecipitated, just before silver chloride begins to precipitate? Ksp for AgCl = 1.8 x 10–10, Ksp for AgBr = 3.3 x 10–13 (a) 0.18% (b) 0.018% (c) 0.0010% (d) 0.00010% (e) 0.0018% b 61. A solution is 1.0 x 10–3 M in Mg(NO3)2 and 1.0 x 10–3 M in Cd(NO3)2. Some solid NaOH is added slowly. What percentage of the Cd2+ originally in solution has precipitated as Cd(OH)2 at the point at which Mg(OH)2 just begins to precipitate? Ksp for Cd(OH)2 is 1.2 x 10–14 and Ksp for Mg(OH)2 is 1.5 x 10–11. (a) 98.12% (b) 99.92% (c) 99.63% (d) 98.73% (e) 99.52% d*62. A solution is 1.0 x 10–4 M in sodium sulfate, Na2SO4, and 1.0 x 10–4 M in sodium selenate, Na2SeO4. Solid barium chloride, BaCl2, is added slowly and one of the two salts, BaSO4 or BaSeO4, begins to precipitate. What percentage of the anion that precipitates first has precipitated at the point at which the second anion begins to precipitate? Ksp for BaSO4 is 1.1 x 10–10 and for BaSeO4 is 2.8 x 10–11. (a) 10% (b) 25% (c) 90% (d) 75% (e) 50% d 63. Solid AgNO3 is added slowly to a solution which is 0.0010 M in NaCl and 0.0010 M in NaBr. The Ksp for AgCl is 1.8 x 10–10 and the Ksp for AgBr is 3.3 x 10–13. What concentration of Br– remains in solution when 50.% of the Cl– has precipitated? (a) 3.6 x 10–7 M (d) 9.2 x 10–7 M (b) 1.2 x 10–8 M (e) 4.2 x 10–9 M (c) 3.3 x 10–10 M Chapter 20 387 b*64. A solution contains 0.10 M Ca2+ and 0.10 M Mg2+. What pH range would result in only Mg(OH)2 precipitating? Ksp for Ca(OH)2 is 6.5 x 10-6 and for Mg(OH)2 is 7.1 x 10-12. (a) (c) (e) 3.85 < pH < 9.82 2.85 < pH < 8.81 8.62 < pH < 11.61 (b) (d) 8.92 < pH < 11.91 9.00 < pH < 13.00 Simultaneous Equilibria Involving Slightly Soluble Compounds c 65. If a solution is 0.10 M in magnesium nitrate and then is also made 0.10 M in aqueous ammonia, what possible precipitate could form? (a) Mg(NO3)2 (d) NH4OH (b) NH4NO3 (e) No possible precipitate (c) Mg(OH)2 d 66. Consider an aqueous solution containing 0.10 M magnesium nitrate and some ammonia. Which response contains all of the following statements that are correct, and no others? Ksp for Mg(OH)2 = 1.5 x 10–11. I. II. III. IV. The solution is saturated with respect to magnesium nitrate. If the concentration of ammonia is high enough, magnesium hydroxide will precipitate. If ammonium chloride is added, the hydroxide ion concentration in solution will be reduced. Nitrate ions, from any ammonium nitrate added, increase the hydroxide ion concentration by reacting with water to form nonionized nitric acid molecules and hydroxide ions. This could cause precipitation of magnesium hydroxide. (a) I, II, and III (d) II and III (b) I and III (e) another combination (c) II and IV d 67. What concentration of aqueous NH3 is necessary to just start precipitation of Mn(OH)2 from a 0.020 M solution MnSO4? Kb for NH3 is 1.8 x 10–5 and Ksp for Mn(OH) 2 is 4.6 x 10–14. (a) 1.4 x 10–5 M (d) 1.3 x 10–7 M c 68. (b) 3.7 x 10–7 M (e) 8.4 x 10–2 M (c) 1.6 x 10–6 M If a solution is to be made 0.010 M in Mg(NO3)2 and 0.20 M in aqueous NH3, how many mol/L of NH4Cl are required to prevent the precipitation of Mg(OH)2 at 25°C? The Kb for aqueous NH3 is 1.8 x 10–5 and the Ksp for Mg(OH)2 is 1.5 x 10–11. (a) 0.054 (b) 0.56 (c) 0.092 (d) 1.8 (e) 0.86 388 b 69. What minimum concentration of ammonium ion (from ammonium chloride) is necessary to prevent the precipitation of Mn(OH)2 from a solution which is to be made 0.030 M in Mn(NO3)2 and 0.030 M in aqueous ammonia? The solubility product for Mn(OH)2 is 4.6 x 10–14, and the ionization constant for aqueous ammonia is 1.8 x 10–5. (a) 0.18 M e 70. (b) 0.44 M (c) 0.54 M (d) 0.72 M (e) 0.88 M If 1.0 liter of solution is to be made 0.010 M in Mg(NO3)2 and 0.10 M in aqueous ammonia, how many moles of NH4Cl are necessary to prevent the precipitation of magnesium hydroxide? The solubility product for Mg(OH)2 is 1.5 x 10–11, and the ionization constant for aqueous ammonia is 1.8 x 10–5. (a) 0.018 mol (d) 0.040 mol (b) 0.016 mol (e) 0.046 mol (c) 0.020 mol b 71. A 2.0-liter volume of a solution is 4.2 x 10–5 M in Mn(NO3)2 and 0.10 M in ammonia. How many moles of NH4NO3 are required to prevent the precipitation of Mn(OH)2? Ksp for Mn(OH)2 = 4.6 x 10–14 and Kb for NH3 = 1.8 x 10–5. (a) 0.62 mol (b) 0.11 mol (c) 0.048 mol (d) 0.24 mol (e) 0.033 mol e*72. A 50.0 mL-sample of 1.8 M aqueous NH3 is mixed with an equal volume of a solution containing 0.010 mol of MgCl2. What mass of NH4Cl must be added to the resulting solution to prevent precipitation of Mg(OH)2? Ksp for Mg(OH)2 = 1.5 x 10–11 and Kb for NH3 = 1.8 x 10–5. (a) 4.4 g (b) 5.7 g (c) 6.2 g (d) 6.6 g (e) 7.1 g Dissolving Precipitates a 73. The solubility of magnesium carbonate, MgCO3, can be increased by acidifying the solution in which MgCO3 is suspended. This is an example of dissolution via _______. (a) formation of a weak electrolyte (b) reduction of the anion (c) oxidation of the anion (d) formation of a complex ion (e) changing an ion to another species which is not a weak electrolyte d 74. Which of the following salts would not be expected to be more soluble in acidic solution than in pure water? (a) Mg(OH)2 (b) ZnS (c) BaCO3 (d) PbCl2 (e) AgF Chapter 20 c 75. Which of the following salts would be expected to be more soluble in acidic solution than in pure water? (a) AgBr e 76. (b) Hg2I2 (c) CaF2 (d) PbCl2 (e) BaSO4 How might one test if PbS has been completely precipitated from a saturated H2S solution? (a) heat the solution (d) add more H2S c 77. 389 (b) add a strong acid (e) add a weak base (c) add a weak acid Consider the following two complex ions of Cd2+ and their dissociation constants, Kd. Kd_____ 2+ [Cd(NH3)4] 1.0 x 10–7 [Cd(CN)4]2– 7.8 x 10–18 Which response contains all the following statements that are true, and no others? I. II. III. IV. [Cd(CN)4]2– is more dissociated in 0.010 M solution than [Cd(NH3)4]2+ is in 0.010 M solution. Adding strong acid to a solution that is 0.010 M in [Cd(NH3)4]2+ would tend to dissociate the complex ion. Adding strong acid to a solution that is 0.010 M in [Cd(CN)4]2– would tend to dissociate the complex ion. If one wished to dissolve a certain weight of insoluble CdI2 by formation of [Cd(CN)4]2– ions by adding either NaCN or HCN, fewer moles of NaCN would be required. (a) I, III, and IV (d) III (b) I and II (e) another combination (c) II, III, and IV b*78. Calculate the number of moles of ammonia needed to dissolve 0.0010 mole of silver chloride in 4000. mL of solution at 25°C. Ksp for AgCl is 1.8 x 10–10 and the dissociation constant for [Ag(NH3)2]+ is 6.3 x 10–8. (a) 0.16 mole (d) 0.28 mole c 79. (b) 0.021 mole (e) 1.5 moles (c) 14 moles Calculate the minimum concentration of NH3 necessary in 1.0 liter of solution to just dissolve 3.0 x 10–3 mole of AgBr at 25°C. Ksp for AgBr = 3.3 x 10–13, Kd for [Ag(NH3)2]+ = 6.3 x 10–8. (a) 6.2 M (b) 8.6 M (c) 1.3 M (d) 0.18 M (e) 0.067 M 390 c*80. What concentration of NH3 must be present in 4.0 x 10–3 M Ag+ to prevent the precipitation of AgCl when [Cl–] reaches 1.0 x 10–3 M? Ksp for AgCl = 1.8 x 10–10 and Kd for [Ag(NH3)2]+ = 6.3 x 10–8. (a) 7.4 x 10– 4 M (d) 1.6 x 10–2 M e 81. (b) 6.1 x 10–3 M (e) 8.5 x 10–1 M (c) 4.5 x 10–2 M The cations in many slightly soluble compounds can form complex ions; this results in the _____________ of the slightly soluble compound as ______________ are formed with molecules and ions such as NH3, CN-, and the halides. (a) precipitation; weak electrolytes (b) dissolution; weak electrolytes (c) dissolution; ions (d) precipitation; coordinate covalent bonds (e) dissolution; coordinate covalent bonds a*82. What is the molar solubility of AgCl in 0.10 M NH3 at 25oC? Ksp is 1.8 x 10-10 for AgCl and Kd for [Ag(NH3)2+] is 5.9 x 10-8. (a) 4.98 x 10-3 M (d) 5.53 x 10-3 M (b) 3.06 x 10-3 M (e) 1.34 x 10-5 M (c) 9.44 x 10-4 b 83. Calculate the concentration of Cu2+ ions in a 0.010 M [Cu(NH3)4]2+ solution at 25°C. Kd for [Cu(NH3)4]2+ = 8.5 x 10–13 (a) 1.3 x 10–3 M (d) 8.7 x 105 M a 84. (c) 6.3 x 10–5 M Calculate the [Zn2+] in a solution which is initially 0.30 M in [Zn(CN)4]2– ions at 25°C. Kd for [Zn(CN)4]2– = 1 x 10–19. (a) 4 x 10–5 M (d) 1 x 10–3 M a 85. (b) 5.1 x 10– 4 M (e) 1.3 x 10–6 M (b) 6 x 10–8 M (e) 3 x 10– 4 M (c) 5 x 10–6 M What is the concentration of free Cd2+ ions in a solution that is 0.30 M in Na2[Cd(CN)4]? Kd for [Cd(CN)4]2– is 7.8 x 10–18. (a) 9.8 x 10–5 M (d) 1.6 x 10– 4 M (b) 6.2 x 10– 4 M (e) 2.8 x 10– 4 M (c) 8.6 x 10–5 M Chapter 20 c 86. 391 Calculate the concentration of cadmium ions in a 0.10 M [Cd(CN)4]2– solution. The complex ion dissociates: [Cd(CN)4]2– Cd2+ + 4CN–, and its dissociation constant is 7.8 x 10–18. (a) 3.0 x 10–5 M (d) 9.8 x 10–5 M (b) 5.7 x 10–5 M (e) 2.2 x 10– 4 M (c) 7.9 x 10–5 M d 87. Calculate the concentration of cyanide ions in a 0.10 M [Cd(CN)4]2– solution. The complex ion dissociates: [Cd(CN)4]2– Cd2+ + 4CN–, and its dissociation constant is 7.8 x 10–18. (a) 1.1 x 10– 4 M (d) 3.2 x 10– 4 M (b) 7.9 x 10–5 M (e) 8.8 x 10– 4 M (c) 9.4 x 10–6 M Conceptual Questions 88. 89. 90. Why is it not correct to call silver chloride an insoluble compound? On the basis of Le Chatelier’s Principle, explain the following observation: addition of ammonium chloride to a beaker containing solid lead hydroxide in water causes the solid to dissolve. Why is the concentration of solid left out the solubility product expression? 91. Addition of HCl and HNO3 both dissolve MnS when added to a saturated aqueous solution . Explain how each helps the dissolution process. Include equations. 92. Barium ions have an approximate LD50 of 80 mg/kg of body mass. Calculate the quantity of barium required to be lethal for a 100 kg man, then calculate the free barium ions contained in a 500 ml, saturated BaSO4 solution dose. From these calculations explain why BaSO4 can be taken orally for intestinal X-rays with no adverse effect.