Ksp
advertisement

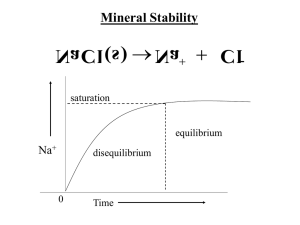
Solubility Product Constant A special case of equilibrium involving dissolving. Solid Positive Ion + Negative Ion Mg(NO3)2 Mg2+ + 2NO3Keq = [Mg2+ ] [NO3-]2 Because the constant is a product of solubility, we call it the solubility product constant Ksp Solubility Product Problems •Given Ksp, find solubility •Given solubility, find Ksp •Find solubility in a solution with a common ion •Predicting precipitation Solubility Product Constant (Ksp) BaF2(S) Ba+2(aq) + 2F-(aq) Write out the equilibrium law expression… Ksp = [Ba+2][F-]2 Solubility Generalizations •All nitrates are soluble •All compounds of the alkali metals are soluble (Li, Na, K, etc.) •All compounds of the ammonium (NH4+) are soluble Given Ksp, Find Solubility What is the solubility of Silver Bromide (Ksp = 5.2 x 10-13) AgBr Ag+ + BrKsp = [Ag+][Br-] = 5.2 x 10-13 Let x = the solubility (x)(x) = 5.2 x 10-13 X2 = 5.2 x 10-13 X = 7.2 x 10-7 Another Example What is the solubility of PbI2 (Ksp = 7.1 x 10-9) PbI2(s) Pb+2 + 2IKsp = [Pb+2][I-]2 = 7.1 x 10-9 Let x = the solubility (x)(2x)2 = 7.1 x 10-9 4x3 = 7.1 x 10-9 (x)(4x2) = 7.1 x 10-9 X = 1.21x10-3 Find Ksp Given Solubility What is the Ksp of Boric Acid, given its solubility of 2.15 x 10-3 Moles/liter? H3BO3 3H+ + BO3-3 Ksp = [H+]3[BO3-3] [3(2.15x10-3)]3 [2.15x10-3] = 5.8 x 10-10 Solubility with a Common Ion What is the solubility of lead iodide (PbI2) in a .15M solution of KI ? PbI2 Pb+2 + 2IKI K+ + IKsp = [Pb+2][I-]2 = 7.1 x 10-9 Solubility with a Common Ion What is the solubility of lead iodide (PbI2) in a .15M solution of KI ? PbI2 Pb+2 + 2IKsp = [Pb+2][I-]2 KI K+ + I- Let x = solubility Then: [Pb+] = x [I-] = .15+2x Ksp = [Pb+2][I-]2 = 7.1 x 10-9 Let x = solubility of PbI2 in the solution Then, [Pb+2] = x [I-] = .15 + 2x Ksp = (x)(.15+2x)2 = 7.1 x 10-9 Ksp = [Pb+2][I-]2 = 7.1 • 10-9 Ksp = (x)(.15+2x)2 = 7.1 • 10-9 (x)(4x2 + .6x +.152) = 7.1 • 10-9 4x3 + .6x2 + .152x = 7.1 • 10-9 4x3 + .6x2 + .0225 – 7.1 • 10-9 = 0 Ksp = [Pb+2][I-]2 = 7.1 x 10-9 Ksp = (x)(.15+2x)2 = 7.1 x 10-9 Assume .15>>2x then, .15+2x .15 Bill Gates is the Richest Man in the World. Woopie! It’s my lucky day! He is worth about… $34 Billion Dollars Now, suppose one day he finds a penny. $34,000,000,000.01 $34 Billion Ksp = [Pb+2][I-]2 = 7.1 x 10-9 Ksp = (x)(.15+2x)2 = 7.1 x 10-9 Assume .15>>2x then, .15+2x .15 Ksp = (x)(.15)2 = 7.1 x 10-9 X = 3.16 x 10-7 moles/liters Predicting Precipitation A student mixes 0.010 mole Ca(NO3)2 in 2 liters of 0.10M Na2C03 solution. Will a precipitate form? Step 1: Write out the dissolving equations Ca(NO3)2 Ca2+ + 2NO3Na2CO3 2Na+ + CO32- Step 2: Determine the most likely precipitate & write out it’s equation. Ca(NO3)2 Ca2+ + 2NO3- Na2CO3 2Na+ + CO32- Recall the solubility generalizations… •All nitrates are soluble •All compounds of the alkali metals are soluble (Li, Na, K, etc.) •All compounds of the ammonium (NH4+) are soluble Step 2: Determine the most likely precipitate & write out it’s equation. Ca(NO3)2 Ca2+ + 2NO3- Na2CO3 2Na+ + CO32CaCO3 Ca2+ + CO32- CaCO3 Ca2+ + CO32- Ksp = [Ca2+ ][CO32-] = 4.7 x 10-9 Step 3: Determine the molar concentrations & calculate the reaction quotient (Q). [Ca2+] = .01 mole/2 liters = .005M [CO32-] = 0.10 M (given) Reaction Quotient (Q) The product of the Ksp equation using the ion concentration before any reaction interaction. If Q > Ksp Then a precipitate will form. Q = [Ca2+ ][CO32-] [Ca2+] = .01mole/2 liters = .005M [CO32-] = 0.10 M (given) Q = (.005)(0.10) = .0005 Q > Ksp a precipitate will form. You try one…. .015 moles of AgNO3 is mixed with 5 liters of .02M NaCl solution. What is the most likely precipitate and will it form? You try one…. .015 moles of AgNO3 is mixed with 5 liters of .02M NaCl solution. What is the most likely precipitate and will it form? AgNO3 Ag+ + NO3- NaCl Na+ + Cl- AgCl Ag+ + Cl- AgCl Ag+ + Cl- Q = [Ag+][Cl-] Q = (.015/5)(.02) = .00006 Look up the Ksp (1.0 x 10-10) Q > Ksp So a precipitate will form!








![K sp = [Pb 2+ (aq)][Cl](http://s2.studylib.net/store/data/005788724_1-fd79e2539544b4374a3f7aa03b8a844b-300x300.png)