Small Scale Ribosome Prep (via Lindahl Lab)
advertisement

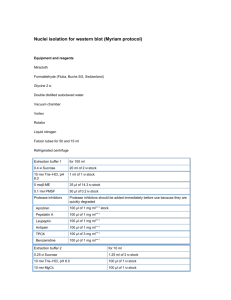
Small Scale Ribosome Prep (via Lindahl Lab) Grow Cells—DAY 1 1. Inoculate 5ml LB with appropriate antibiotics and grow ON 2. In AM, inoculate 200ml LB with appropriate antibiotics to OD450=0.05, grow cells to OD450=1.5-2.0. Take OD450 readings every 30min and plot on log paper 3. At OD450=1.4-2.0 harvest cells in a 250ml centrifuge bottle and spin at 8K rpm, 10min. 4. Resuspend pellet dry, add 1ml Buffer A; resuspend well and transfer to 2ml centrifuge tube. 5. Spin @10K rpm for 5min, decant, resuspend in 1ml Buffer A 6. Split into 2 tubes and add 100ul 15mg/ml lysozyme (fresh) to each tube, mix, incubate on ice 3min, flash freeze at -80C. Mini prep—DAY 2 Prepare lysates – 1. Remove tubes from -80 and slow thaw in ice-water bath (take ~2hours) 2. Clarify lysate by spinning in Beckman Optima Ultracentrifuge in MLA 130 rotor at 22K rpm for 30min using open top 11x34 mm tubes (#343778) either use immediately or transfer supernatant to 2ml tube flash freeze and store at -80C (reclarify by spinning at 13K rpm for 5min) Pellet ribosomes – DAY 3 1. Transfer clarified lysate to 2ml sealable tube for MLA 130, top off with Buffer A as needed and seal. Place in rotor with spacers 2. Spin at 40K rpm for 4 hours, resuspend in 100ul Buffer A, rock overnight at 4C. 3. In AM, transfer to 1.5ml tube, spin at 13K 10min at 4C. Transfer to new tube and measure OD260 (1:100 dilution). 4. Store ribosomes at -80C. SOLUTIONS: Buffer A (autoclave) HEPES-KOH pH 7.5 MgCl2 NH4Cl Mercaptoethanol working con 20mM 60mM 30mM 6mM Stock 2M 1M solid 1L 30X 300ml 180ml 48.2g To make 100ml 1X Buffer A: 3.3ml 30X Buffer A qs to 100ml with water, add 27ul mercaptoethanol store at 4C HEPES Salt Wash Buffer HEPES MgCl2 NH4Cl Stock 2M 1M solid 100ml 1ml 3ml 5.4g MET d2H20 pure 40ul to 100ml