Spectrophotometry
advertisement
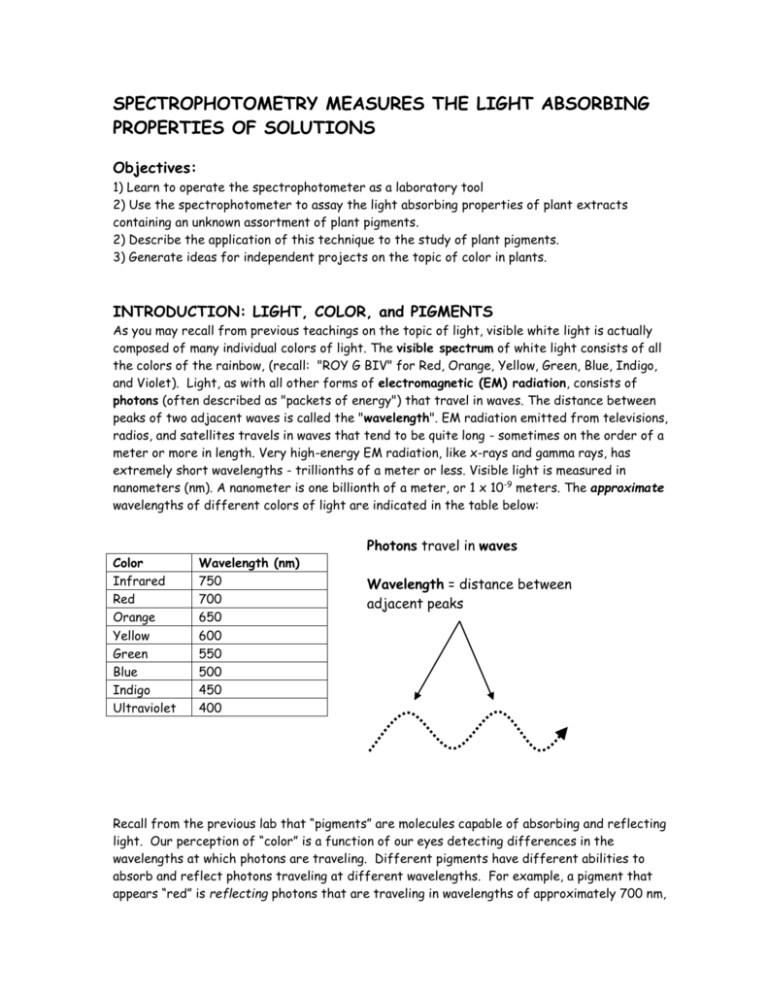
SPECTROPHOTOMETRY MEASURES THE LIGHT ABSORBING PROPERTIES OF SOLUTIONS Objectives: 1) Learn to operate the spectrophotometer as a laboratory tool 2) Use the spectrophotometer to assay the light absorbing properties of plant extracts containing an unknown assortment of plant pigments. 2) Describe the application of this technique to the study of plant pigments. 3) Generate ideas for independent projects on the topic of color in plants. INTRODUCTION: LIGHT, COLOR, and PIGMENTS As you may recall from previous teachings on the topic of light, visible white light is actually composed of many individual colors of light. The visible spectrum of white light consists of all the colors of the rainbow, (recall: "ROY G BIV" for Red, Orange, Yellow, Green, Blue, Indigo, and Violet). Light, as with all other forms of electromagnetic (EM) radiation, consists of photons (often described as "packets of energy") that travel in waves. The distance between peaks of two adjacent waves is called the "wavelength". EM radiation emitted from televisions, radios, and satellites travels in waves that tend to be quite long - sometimes on the order of a meter or more in length. Very high-energy EM radiation, like x-rays and gamma rays, has extremely short wavelengths - trillionths of a meter or less. Visible light is measured in nanometers (nm). A nanometer is one billionth of a meter, or 1 x 10-9 meters. The approximate wavelengths of different colors of light are indicated in the table below: Color Infrared Red Orange Yellow Green Blue Indigo Ultraviolet Wavelength (nm) 750 700 650 600 550 500 450 400 Photons travel in waves Wavelength = distance between adjacent peaks Recall from the previous lab that “pigments” are molecules capable of absorbing and reflecting light. Our perception of “color” is a function of our eyes detecting differences in the wavelengths at which photons are traveling. Different pigments have different abilities to absorb and reflect photons traveling at different wavelengths. For example, a pigment that appears “red” is reflecting photons that are traveling in wavelengths of approximately 700 nm, while at the same time absorbing photons that are traveling in wavelengths that are both shorter and longer than 700 nm. We perceive the color “red” because only the photons traveling at 700 nm are reflected back to our eyes (our eyes cannot detect the photons that are absorbed by a pigment). How do we explain the perception of “black” and “white” pigments in terms of reflectance/absorbance of light? Colors vary among pigments because different pigments absorb and reflect different wavelengths of light, as well as different amounts of the same wavelength. For example, back to our discussion of red – not all reds appear the same. Some reds are lighter or darker than others, or they may have different qualities resulting from undertones, hues, or casts of other colors, such as blues or yellows. ASSAYING THE LIGHT-ABSORBING PROPERTIES OF SOLUTIONS WITH THE SPECTROPHOTOMETER A spectrophotometer is a laboratory instrument that allows us to examine the light-absorbing properties of a solution. Solutions are mixtures of different kinds of molecules. Solutions consist of solutes (molecules that dissolve into a solution) and solvents (the collection of molecules that comprise the substance that the solutes dissolve in). Molecules, whether in solutes or solvents, absorb and reflect light and different molecules have different abilities to absorb and reflect light at different wavelengths. Additionally, two different molecules might both be able to absorb the same wavelength of light (for example, red light), but differ in the quantity of light that each absorbs. The spectrophotometer allows us to select a single wavelength of light, pass it through a solution, and determine the total amount of light of that wavelength that is absorbed by the solution. It does NOT tell us which molecules are accounting for the light absorption, only how much light of a given wavelength is absorbed by all the molecules in the solution. If we are interested in the light absorbing properties of the solute alone, we must “blank” the spectrophotometer with a solution containing solvent only to eliminate absorbance due to molecules that make up the solvent (more on this later). Similarly, if we want to compare the light absorbing properties of pigments (solutes) contained in different plant tissues, we must ensure that our extracts are prepared in the same way (e.g., using the same solvent in the same quantity). Get to know your spec! After becoming acquainted with the various parts and dials, try the following exercise to familiarize yourself with the spectrophotometer. 1) Cut a strip of white paper that exactly fits the length and width of a "cuvette" (the name of the specialized tubes that go into a spectrophotometer). Insert a cuvette with the paper into the cuvette holder in the spectrophotometer. Align the white stripe on the cuvette with the line on the cuvette holder.ONLY cuvettes should be inserted into the spectrophotometer. Never insert a standard test tube into the spec! 2) Leaving the sample holder cover open, place a cylinder of black paper around the opening that you will look into (think: play telescope). 3) Set the wavelength to 620 nm. Adjust the position of the cuvette tube to maximize the amount of red light you see on the side of the paper. 4) While one person looks into the cuvette, have another person adjust the wavelength setting. Record the range of wavelengths at which you see each of the colors of the rainbow (Red, Orange, Yellow, Green, Blue, Indigo, Violet). This exercise is to 1) account for variation among different machines in the wavelengths displayed and the color of light transmitted and, 2) to illustrate that adjusting the wavelength setting will, in fact, change the color of light transmitted through the cuvette. PLANT PIGMENT ANALYSIS WITH THE SPECTROPHOTOMETER PART 1. PREPARE YOUR SOLUTIONS – AN ORIGINAL (100%) EXTRACT AND DILUTION SERIES Plant pigments are usually sequestered inside membrane-bound organelles in plant cells (e.g., chlorophyll in chloroplasts, carotenes in chromoplasts, and anthocyanins in vacuoles). In order to release these pigments into a solution that can be assayed by the spectrophotometer, we must first destroy the plant cells and organelles – meaning, break up the membranes so that the pigments can be released from the organelles (plastids and vacuoles) into solution. We will do this flash-freezing the tissue with liquid nitrogen, then grinding the plant material with a mortar and pestle. Note: The actual amount of plant material used to prepare the extract is quite small. If you’re interested in assaying the pigments of a large flower, fruit, or leaf, you may find it best to first prepare a homogenate. Homogenize the larger sample of plant tissue by chopping into very fine pieces using a blender, food mill, or by hand, then mixing thoroughly before removing a subsample of the homogenate of the appropriate weight for preparing the extract. Prepare your “original” plant extract (100%): 1) Tare a weigh boat and weigh 0.5 g of your plant material. Record the exact weight in your lab notebook. Transfer all the weighed plant material into a mortar (the basin; the pestle is the grinding device). 2) Add a small amount of liquid nitrogen to the mortar and grind quickly by hand with the pestle until your plant material resembles a fine powder. Continue adding small amounts of liquid N if your sample begins to thaw. WARNING: Though it’s way cool and looks like a lot of fun, liquid N is dangerous and can cause serious harm when mishandled!! Wear safety goggles and protective gloves when handling! 3) Measure 15 ml ethanol into a graduated cylinder. Using a transfer pipet, add approx. 5-10 ml of the 15 ml ethanol to your mortar and continue grinding to extract the pigments into solution. Reserve the remainder of the 15 ml ethanol to rinse the plant material adhering to the mortar and pestle. 4) Line a glass funnel with a filter paper cone and place the neck of the funnel into a 25ml test tube or flask labeled “100%”. Filter the solid particles out of your sample solution by pouring the extract into the lined funnel. Use the remainder of your 15 ml ethanol to rinse the mortar and pestle into the funnel. ALL of the 15 ml ethanol needs to go into the sample extract. THIS IS YOUR ORIGINAL PLANT EXTRACT (100%). Prepare a dilution series (50%, 25%, 12.5%): 1) Collect 3 smaller (10 - 15 ml) test tubes (not cuvettes), and label as follows: 50%, 25%, 12.5% 2) Make the 50% solution: Transfer 4 ml of 100% solution to the tube labeled “50%” and add 4 ml ethanol to it. Mix thoroughly by covering the test tube opening with a finger and gently tapping the base to create a vortex. 3) Make the 25% solution: Transfer 4 ml of 50% solution to the tube labeled “25%” and add 4 ml ethanol to it. Mix thoroughly by covering the test tube opening with a finger and gently tapping the base to create a vortex. 4) Make the 12.5% solution: Transfer 4 ml of 25% solution to the tube labeled “12.5%” and add 4 ml ethanol to it. Mix thoroughly by covering the test tube opening with a finger and gently tapping the base to create a vortex. Test Tube Label 100 50 25 12.5 Solution original plant extract 4 ml 100% solution 4 ml 50% solution 4 ml 25% solution Ethanol 0 ml 4 ml 4 ml 4 ml Final Concentration 100% 50% 25% 12.5% PART 2. CHARACTERIZE THE LIGHT-ABSORBING PROPERTIES OF YOUR PLANT EXTRACT - GENERATE AN ABSORBANCE SPECTRUM AND DETERMINE AMAX THE WAVELENGTH AT WHICH YOUR PLANT EXTRACT ABSORBS THE MAXIMUM AMOUNT OF LIGHT epxlal 1) Prepare a sample cuvette. Label a cuvette tube with a pencil or china marker (do not use a Sharpie or other permanent marker) to identify your sample. Use a transfer pipet to add some of your 100% plant extract to the cuvette tube. The cuvette should be filled to within ½ - 1 inch of the white dot on the cuvette tube. (Note: If you find that your 100% solution is too concentrated to be read by the spectrophotometer, use your 50% solution for this exercise.) 2) Prepare a blank cuvette. Label another cuvette tube “blank” and fill with ethanol to within ½ - 1 inch of the white dot. A "blank" is a sample that is prepared with all the same components as the unknown sample, except for the molecule of interest. In this case, the molecules of interest are the pigments contained in the plant extract you prepared. As a result, your blank will contain only ethanol since that is the only thing present in the unknown extract other than the plant pigments we isolated. 3) Calibrate the spectrophotometer. After the spectrophotometer has warmed up for at least 15 minutes, it will need to be adjusted (calibrated) to ensure that it reads from the entire range of its scale. a. Set the wavelength to 450nm. Locate the filter lever at the lower left of the spectrophotometer and move it to the “340-599nm” position. Rotate the “wavelength” dial until the display reads 450nm. b. Set the empty chamber to 0% Transmittance. Push the white button labeled “Mode” until “Transmittance” is highlighted on the display. With the sample chamber empty (no cuvette) and cover closed, adjust the transmittance to 0% by rotating the dial on the left labeled “0%T”. (When there is no cuvette in the chamber, the light path is blocked by a physical barrier so that no light reaches the sensor - thus, 0% light is transmitted.) 4) Blank the Spectrophotometer. Press the “Mode” button until the display reads “Absorbance”. Use a kimwipe to clean the surface of the Blank cuvette - there should be no fingerprints, smears, or other residue on the surface. Insert the cuvette with blank into the chamber, align the white line on the cuvette with the line in the sample chamber, and close the chamber door. Adjust the absorbance to 0% by rotating the dial on the right labeled “100%T/0A”. *Molecules in the ethanol blank absorb light, but we are only interested in the lightabsorbing properties of the plant pigments in our extract, not the ethanol solvent. "Blanking" with a cuvette of solvent "subtracts" the light absorption due to non-target molecules – in our case, ethanol. We have calibrated the blank to transmit 100% of the light passing through it. This means than none of the light, or 0% of the light is being absorbed. Absorbance and Transmittance are inversely related to each other. 5) Read the unknown. Use a kimwipe to clean the surface of the cuvette containing your 100% sample extract to remove any residue. Insert the sample into the sample chamber, close the door, read and record the Absorbance of your sample. This value (the A450 or, the Absorbance at 450nm) is the amount of 450 nm wavelength light that can be absorbed by the pigments contained in your extract. If your sample causes the display to blink “1.999”, then your solution is too concentrated and light cannot pass through. Simply remove your sample cuvette and use your 50% solution for the remainder of the exercise. 6) In increments of 25nm, read the absorbance of your sample from 450 – 800nm. Change the wavelength setting to 475 nm and reinsert the blank. Adjust the dial until the blank reads 0 absorbance. Insert your sample and record the absorbance value for 475 (A475). Continue the sequence of: 1) change wavelength, 2) blank, 3) read sample absorbance, for your sample at wavelength increments of 25 nm for all wavelengths from 450 800nm. Record all your data in your lab notebook. Note: when you reach 600nm, you will need to shift the filter lever to the “600-950nm” position. 7) Draw the absorbance spectrum. Plot your data in graphical form with wavelength along the x-axis and absorbance on the y-axis. Does your sample have more than one peak? 8) Determine the Amax for your sample. The Amax is the wavelength at which your sample absorbs maximally. You will determine this by looking at your absorbance spectrum and noting the wavelength which has the highest absorbance value. PART 3. GENERATE A STANDARD CURVE TO ESTIMATE CONCENTRATION As you increase the concentration of a substance, you increase the number of molecules present in the same volume of solution. As a result, more molecules of light-absorbing substance will cause more light to be absorbed. Because increasing concentration results in increasing absorbance, we can use spectrophotometry as a method to estimate the concentration of a molecule of interest in an unknown sample. Use the dilution series you prepared earlier to observe the relationship between concentration and absorbance. epxlal 1) Set the wavelength of the spectrophotometer to the wavelength that corresponded to the Amax for your solution. 2) Blank the spectrophotometer to read 0% Absorbance. 3) Read each of your sample dilutions (100%, 50%, 25%, and 12.5%) at this wavelength (Amax) and record the absorbance for each. Note: In this case, you will be reading all the tubes at the same wavelength. Since you are not changing wavelength, you do not need to reblank the machine prior to each reading. However, you should reblank the machine whenever it appears that the display is "drifting" between readings, or if you're taking several readings over more than just a few minutes. PART 4. ESTIMATE THE CONCENTRATION OF CHLOROPHYLLS A AND B USING KNOWN ABSORBANCE VALUES Set the wavelength setting on the spectrophotometer to 663 nm (Amax for chlorophyll a). Blank the spectrophotometer with ethanol, then read the absorbance of your 100 % sample extract. Repeat for chlorophyll b by blanking and reading your sample at 645 nm (Amax for chlorophyll b). Estimate the concentrations of chlorophyll a and chlorophyll b per gram of fresh weight plant tissue using the equations below: *Chlorophyll a (mmol/L) = [Abs at 663nm] / [(75.05 L/mmol-cm)a x (1.17 cm)c] grams fresh plant tissue used in extract *Chlorophyll b (mmol/L) = [Abs at 645nm] / [(47.0 L/mmol-cm)b x (1.17 cm)c] grams fresh plant tissue used in extract a = extinction coefficient for chlorophyll a b= extinction coefficient for chlorophyll b c = length of light path for Spectronic 20 Chlorophyll concentration is measured in units of mmol/L/g (equivalent to umol/mL/g). POSTLAB QUESTIONS: 1. Why do you need to reblank the spectrophotometer every time you change the wavelength setting? 2. Plot the absorption spectrum for your plant extract. Be sure to include a title and label the axes! 3. What is the Amax for your sample? Approximately what color of light does this correspond to? 4. Compare and contrast the Amax with another point on your absorption spectrum that has a low absorption value. Explain what is happening at these two points in terms of the light absorbing properties of your plant extract – include in your explanation: wavelength, color, absorption, and reflection. 5. Plot the “standard curve” you generated as a result of your serial dilutions. Be sure to include a title and label the axes. Determine the concentration of an unknown with an absorbance value given to you by your TA – illustrate on your standard curve.