File
advertisement

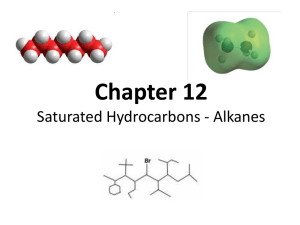
Standard Grade Chemistry Summary Notes Topic 6: Structures & Reactions Of Hydrocarbons Learning Outcomes General Alkanes are a subset of the set of hydrocarbons. Alkanes have an ‘-ane’ ending in their name. The first eight alkanes are : methane, ethane, propane, butane, pentane, hexane, heptane and octane. You should be able to construct for straight chain alkanes (C1 to C8) full and shortened structural formulae and molecular formulae given the name You should be able to name an alkane (C1 to C8) given its molecular, shortened or full structural formulae. The general formula for alkanes is CnH2n+2 and you should be able to use this to work out the structural formula of a named alkane. Alkenes are a subset of the set of hydrocarbons. Alkenes have an ‘-ene’ ending in their name. The first five alkenes are : ethene, propene, butene, pentene, hexene You should be able to name an alkene ( to C6) given its molecular, shortened or full structural formulae. You should be able to construct for straight chain alkenes (C1 to C6) full and shortened structural formulae and molecular formulae given the name The general formula for alkenes is C nH2n and you should be able to use this to work out the structural formula of a named alkene. You should be able to identify from structural formula whether a hydrocarbon is an alkane or an alkene. A saturated hydrocarbon contains only carbon to carbon single bonds. Alkanes are saturated hydrocarbons. Unsaturated hydrocarbons contain carbon to carbon double bonds. Alkenes are a subset of the set of unsaturated hydrocarbons. It is possible to distinguish between an unsaturated and a saturated hydrocarbon using bromine (solution). Alkenes decolourise bromine solution rapidly, alkanes do not. Alkanes are formed from the reaction of an alkene with hydrogen. An addition reaction occurs when a diatomic molecule adds on across a C=C double bond in an alkene. The reactions between alkenes and hydrogen and bromine are addition reactions. Fractional distillation of crude oil yields more long chain hydrocarbons than are useful for present-day industrial purposes. Cracking is an industrial method for producing smaller, more useful molecules. State that cracking produces smaller hydrocarbons, some of which are unsaturated. Credit A homologous series is a series of compounds that - fit a general formula - have the same chemical properties - show a gradual change in physical properties, like boiling points and viscosity The alkane family is an example of an homologous series As the number of carbon atoms increases in the formula then the molecules become bigger in size. The bigger the molecules, the more energy is required to get the molecules to move about rapidly. This explains why the alkanes with the smallest molecules have the lowest boiling points and those with the largest molecules have the higher boiling points. The first 4 cycloalkanes are ; cyclopropane, cyclobutane, cyclopentane and cyclohexane. The cycloalkane family is an example of a homologous series. Isomers have the same molecular formula but have different structural formulae. The alkene family is an example of a homologous series. A catalyst allows an addition reaction to take place at a lower temperature. Cracking produces a mixture of saturated and unsaturated products as the number of carbon and hydrogen atoms in the cracked alkane must equal the number of carbon and hydrogen atoms in the products. Alkanes Hydrocarbons are compounds of hydrogen and carbon only. Methane is the simplest hydrocarbon. There are many different hydrocarbons due to carbon being a very special element. Carbon is special because : Carbon can form bonds with itself to form chains of carbon atoms Carbon can form bonds with itself to form chains of carbon atoms with branches off the main chain Carbon can form bonds with itself to form rings of carbon atoms No other element can do this as well as carbon. The hydrocarbons are divided into families. The simplest family of hydrocarbons are the alkanes. Methane is the simplest member of the alkane family. All of the fractions obtained by the fractional distillation of crude oil contain alkanes. Alkanes are found in many every day products such as petrol, diesel, aviation fuel, paraffin, camping gases, lighter fuel, lubricating oils, candle wax, tar etc. Properties Of Alkanes highly flammable – burn in a plentiful supply of oxygen to form carbon dioxide and water pH = 7 insoluble in water very slowly decolourise bromine solution Alkane Series Methane, and the mixture of alkanes contained in paraffin, are members of a large family of alkanes. The name of the alkane compounds always end in –ane. The first part of the alkane name indicates the number of carbon atoms there are in the molecule. For example BUT ANE means there are four carbons in the alkane means the compound is an alkane It is important that you know the prefixes (first part of the name) for molecules with up to eight carbon atoms. Number of carbon atoms 1 2 3 4 Prefix methethpropbut- Number of carbon atoms 5 6 7 8 Prefix penthexheptoct- Here is a table of data on some members of the alkanes. Alkane methane ethane propane butane pentane hexane heptane octane Chemical Formula CH4 C2H6 C3H8 C4H10 C5H12 C6H14 C7H16 C8H18 Number of carbon atoms 1 2 3 4 5 6 7 8 Boiling point (oC) -161 -89 -42 0 36 69 98 126 State at 25oC gas gas gas gas liquid liquid liquid liquid Boiling point is an example of a physical property of a compound. Physical properties are those which can be measured in some way. Chemical properties are those concerning the way the substance behaves e.g. burns to form carbon dioxide and water. The boiling point of an alkane can be measured by distillation. You should see from the above table of data on the alkanes that the boiling point increases as the number of carbon atoms increases. As the size of the molecule increases, more energy is needed to separate them. Since boiling involves separating molecules, the boiling point must increase as the alkane molecule gets longer? There are also trends in other properties of the alkanes. Viscosity, flammability and sootiness of the flame, all vary according to the number of carbon atoms contained in the molecules. Although there are trends in some properties of the alkanes they all undergo the same type of reactions. All the alkanes burn to form carbon dioxide and water, although the shorter change molecules burn more easily. All the alkanes only slowly decolourise bromine solution. The alkanes are said to be chemically similar. The chemical formula of the alkanes varies by –CH2 as you go up the series. This common difference between members of the alkanes means that the family can be represented by a general formula : CnH2n+2 where n is the number of carbon atoms A family of compounds, like the alkanes, which are chemically similar and have the same general formula are called a homologous series. Alkane Structures The general formula of the alkanes, CnH2n+2 allows us to work out the molecular (chemical) formula of any alkane. e.g. pentane pent- indicates that the alkane has 5 carbons. Therefor, molecular formula of pentane is C5H (2X5+2) i.e. C5H12 The molecular formula does not tell us anything about the structure of the molecule. Each carbon forms four covalent bonds (valency of carbon is 4), and each hydrogen forms one covalent bond (valency of hydrogen is 1). We also know from earlier in this topic that carbon can form bonds with itself to form chains of carbons. A picture of a methane molecule would show it to have a tetrahedral structure because the hydrogen atoms sit at the corners of a shape called a tetrahedron. For ease of drawing the molecule can be represented in this ‘flattened-out’ way. Drawings can be made of larger straight chain molecules. These drawings are called full (or extended) structural formulae. The easiest way to draw the full structural formula of an alkane is to begin by drawing the correct number of carbons in a line, then join on the hydrogens, remembering that each carbon must have four bonds, but each hydrogen only one bond. e.g. pentane is C5H12 Begin by drawing the 5 carbons atoms in a line. C–C–C–C–C Now add the hydrogens, remembering each carbon must have 4 bonds and each hydrogen 1 bond. H H H H H H–C–C–C–C–C–H H H H H H Shortened Structural Formulae The full structural formula of pentane (C5H12) is H H H H H H–C–C–C–C–C–H H H H H H but its shortened structural formula is CH3CH2CH2CH2CH3 Using the full structural formula, the number of hydrogens on each carbon is counted. In the example of pentane, the first carbon has three hydrogens attached, so you write down CH3, the next carbon has two hydrogens attached (CH2) and so on. Often, when there a lot of consecutive CH2 groupings to write down, brackets are used e.g. for pentane, instead of writing CH3CH2CH2CH2CH3, the shortened structural formula can be written as CH3(CH2)3CH3 Isomers Full structural formulae can be drawn in different ways for the same molecules with the same molecular formula. Consider the following molecules. Both have the molecular formula C5H12. H H H H H H C–C–C–C–C–H H H H H H H H H H and H–C–C–C–C–H H H H H–C–H H Compounds with the same molecular formula but different structural formula are called isomers. Cycloalkanes Carbon atoms can join with other carbon atoms to form rings. The hydrocarbons which have carbons joined in rings are called cycloalkanes. ‘Cyclo’ means joined in a ring. The smallest number of carbon atoms which can join to form a ring is three. The first member of the homologous series is therefore cyclopropane. Since each carbon in the ring is joined to neighbouring carbons, and since the valency of carbon is four, each carbon in the ring is also joined to two hydrogen atoms. The following table shows the names and chemical formulae of the first four members of the cycloalkanes and the structural formula of cyclobutane. Cycloalkane cyclopropane cyclobutane cyclopentane cyclohexane Molecular formula C3H6 C4H8 Structural formula H H H–C–C–H H–C–C–H H H C5H10 C6H12 The general formula of the cycloalkanes is CnH2n. The cycloalkanes have very similar properties to the alkanes. i.e. they burn in the plentiful supply of oxygen to form carbon dioxide and water, they only very slowly decolourise bromine solution, they are insoluble in water, and they have a pH = 7. Cracking Crude oil undergoes fractional distillation to separate it into different fractions. These fractions are mixtures of hydrocarbons and have many different uses in the modern world. Unfortunately the fractional distillation process does not produce every fraction in the correct quantity to match the demand for its various uses. In general terms, fractional distillation produces more of the long chain hydrocarbons than are needed and not enough of the short-chain hydrocarbon fractions. Because of this greater demand for the shorter-chain hydrocarbons chemists ‘chop up’ some of the surplus longer molecules from the heavier fractions to get smaller molecules. This process is called cracking. In cracking heat is used to break up the long chains. However, the use of a catalyst can allow the process to take place at a lower temperature, as well as speeding up the process. Cracking can be demonstrated in the laboratory. The steel wool catalyst is heated strongly. The heat passes along the boiling tube and causes the liquid paraffin to evaporate and pass over the heated catalyst. This causes the long chain hydrocarbons in the paraffin to break up into shorter chain molecules. The shorter chain gaseous molecules are collected as shown. The gas collected can be shown to burn and to immediately decolourise bromine solution. The fact that the gas collected by cracking the liquid paraffin immediately decolourises bromine solution indicates that a different type of molecule has been formed. This type of molecule belongs to another hydrocarbon family of compounds called the alkenes. The alkenes are special because they have a carbon-to-carbon double bond between two of the carbons in the molecule. Hydrocarbons with a carbon-tocarbon double bond, like the alkenes are described as being unsaturated. Alkanes and cycloalkanes are both described as saturated because the molecules in these families contain only carbon-to-carbon single bonds. The cracking of long-chain alkane molecules to produce two shorter chain molecules must result in one of the smaller molecules being unsaturated because there are not enough hydrogen atoms available to form two saturated molecules. C7H16 C5H12 + saturated alkane C2H4 unsaturated alkene Alkenes The alkenes are a homologous series of unsaturated hydrocarbons. They result from the catalytic cracking of long chain hydrocarbon fractions. They are said to be unsaturated because they contain one carbon-to-carbon double bond between two of the carbons in the chain. Since the presence of the double bond requires two carbons, the first member of the homologous series of alkenes is the two carbon molecule ethene. This is the structure of ethene H H C=C H H The following table gives information on the first few members of the alkenes. Alkene ethene propene butene pentene hexene Number of carbons 2 3 4 5 6 Molecular formula C2H4 C3H6 C4H8 C5H10 C6H12 Boiling Point (oC) -104 -47 -6 +30 +63 State at 25oC gas gas gas liquid liquid The general formula of the alkene, CnH2n. Note that the general formula of the alkenes is the same as the general formula of the cycloalkanes. However the alkenes and the cycloalkanes are different in structure and also in some properties. It should also be noted that since alkenes and cycloalkanes with the same number of carbons e.g. butene and cyclobutane have the same molecular formula but different structures, that they are isomers. Alkene Reactions After testing hexane with bromine, we can see that it is easy to tell alkenes from alkanes. Alkene Quick decolourisation of bromine solution. Alkane Slow decolourisation of bromine solution. The reason for the difference in reaction towards bromine must lie with their difference in structure. The double bond in the alkenes is responsible for their quick reaction with bromine. The double bond breaks and the bromine atoms simply add on. Example H H C=C + H H Br – Br H H Br – C – C – Br H H ethene bromine dibromoethane This type of reaction is typical of unsaturated hydrocarbons such as the alkenes and is called an addition reaction. Alkenes can also react with hydrogen in an addition reaction to form the corresponding alkane. The hydrogen atoms add across the carbon-to-carbon double bond converting the unsaturated alkene into the saturated alkane. Addition reactions do not occur with alkanes. Since each carbon in an alkane is already joined to four other atoms, some atoms have to be removed before others can join on. This is a much slower type of reaction and is called a substitution reaction.