Au8 luminescence spectra calculation
advertisement

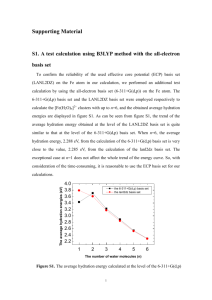
Supporting document Aqueous-based synthesis of atomic gold clusters: Geometry and optical properties S. Rath1,a), S. Nozaki1 , D. Palagin2, V. Matulis2, O. Ivashkevich2, and S. Maki3 1 Department of Electronic Engineering, The University of Electro-Communication 1-5-1 Chofugaoka, Chofu-Shi, Tokyo 182-8585, Japan 2 Research Institute for Physical Chemical Problems, Belarusian State University, 14 Leningradskaya Street, Minsk, 220030, Republic of Belarus 3 Department of Applied Physics and Chemistry, The University of Electro-Communication 1-5-1 Chofugaoka, Chofu-Shi, Tokyo 182-8585, Japan 1. Calculations of absorption spectra To calculate the optical absorption, the geometries of clusters were optimized using the B3LYP1 (Becke three-parameter Lee-yang-Parr) functional and LANL2DZ (Los Alamos National Laboratory 2- double Zeta) basis set2, and the optical spectra were calculated using the TDB3LYP/LANL2DZ level of theory3. Table I. B3LYP/LANL2DZ calculated relative energies of the optimized Au8 structures. The energy of most stable planar star shaped cluster is taken as a reference point. Structure number Structure name Relative energy, eV 1 planar star shaped 0.00 2 planar hexagon+1 shaped 0.54 3 planar boat shaped 0.71 4 3D tetrahedral shaped 0.78 5 3D C2v pyramid shaped 0.75 1 Fig. 1. TD-B3LYP/LANL2DZ calculated optical absorption spectrum of the star shaped Au8 cluster. Inset is the B3LYP/LANL2DZ optimized geometry of the star shaped Au8 cluster. Fig. 2. TD-B3LYP/LANL2DZ calculated optical absorption spectrum of the boat shaped Au8 cluster. Inset is the B3LYP/LANL2DZ optimized geometry of the boat shaped Au8 cluster. 2 Fig. 3. TD-B3LYP/LANL2DZ calculated optical absorption spectrum of the tetrahedral shaped Au8 cluster. Inset is the B3LYP/LANL2DZ optimized geometry of the tetrahedral shaped Au8 cluster. Fig. 4. TD-B3LYP/LANL2DZ calculated optical absorption spectrum of the pyramid shaped Au8 cluster. Inset is the B3LYP/LANL2DZ optimized geometry of the pyramid shaped Au8 cluster. 3 Table II. B3LYP/LANL2DZ calculated and experimental absorption spectra features for hexagon+1 shaped Au8 cluster Peak number Peak position, nm Calculated Experimental 1 371.3 375.5 2 278.7 257.2 3 233.1 226.4 The absorption spectrum calculated for hexagon+1 structure shows three distinct electronic transitions at about 371.3, 278.7 and 233.1 nm. The peak wavelength of 371.3 nm is lower by 4.2 nm than that of the spectrum measured for the as-prepared or resin-treated sample, 375.5 nm. Since the surfactant does not seem to affect the peak wavelength in the measured optical absorption spectra, the discrepancy may be due to inaccuracy in calculation. The wave functions of the excited states considerably change from that of the ground state, and the change is more significant for the higher-energy states. Nevertheless, the absorption spectrum calculated for an Au8 cluster with the planar hexagon+1-shaped geometry most agrees in the peak wavelengths with the measured spectrum among those of Au8 clusters with various geometries. 2. Calculations of emission spectrum We suggest the following way to predict the emission spectrum. The geometry optimization of Au8 cluster in singlet excited state has been carried out using TDB3LYP/LANL2DZ1-3 method. The optimized structure has been used B3LYP/LANL2DZ simulation of emission spectrum. References 1 P. J. Hay, W. R. Wadt, J. Chem. Phys. 82, 299 (1985). 2 R. E. Stratmann, G. E. Scuseria, M. J. Frisch, J. Chem. Phys. 109, 8218 (1998). 3 A. D. Becke, J. Chem. Phys. 98, 5648 (1993). 4 for TD-