File wheres al 9 25 15
advertisement

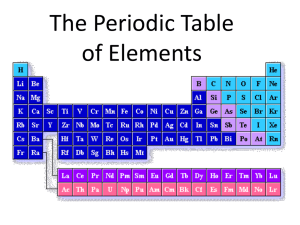
TEKS 8.5A describe the structure of atoms including the masses, electrical charges and locations, of protons and neutrons in the nucleus and electrons in the electron cloud TEKS 8.5B identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity TEKS 8.5C interpret the arrangement of the Periodic Table, including groups and periods WHERE’S AL? Objectives: Interpret the arrangement of the Periodic Table. Describe the structure of atoms. Materials: blank paper cousin’s page glue scissors Teacher Directions: In advance, copy one cousin page for each student. These may be laminated and cut apart for repeated use. Students will arrange by any property that is counted; for example, the arms or the fingers. Provide hints for those that become frustrated. Hints: arrange from smallest to largest with the smallest at the top and the largest at the bottom. “WHERE’S AL?” Discussion Questions 1. energy levels electrons group number /valence electrons atomic mass reactivity members of the same group number of arms number of fingers number of hairs on head size of body smile or lack of smile clothing type 2. Some cousins are smiling because their outermost energy level is full or nearly full. Those that are not full are reactive. 3. See Cousins page 4. The Periodic Table is arranged from left to right in order of increasing atomic number or by increasing atomic mass. Groups on the Periodic Table have similar properties and the same number of valence electrons. The number of energy levels increase as you move down a group. (Answers will vary)—Elements go from reactive on the left to nonreactive on the right of the Periodic Table. Arranged by increasing number of protons. 5. Answers will vary, but student’s could draw different hats, skirts, flowers, shoes etc. made of +’s to show the positive charge on the protons as well as Al’s unique identity. Where’s Al Answer Key Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω TEKS 8.5A describe the structure of atoms including the masses, electrical charges and locations, of protons and neutrons in the nucleus and electrons in the electron cloud TEKS 8.5B identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity TEKS 8.5C interpret the arrangement of the Periodic Table, including groups and periods WHERE’S AL? Objectives: Interpret the arrangement of the Periodic Table Describe the structure of atoms Directions: 1. Cut out the cousins and place them in front of you. 2. Use clues from the letter below to arrange the cousins into their families. 3. One picture, Cousin Al, is missing. Once you determine his proper position, predict what he looks like and sketch him in the correct position. 4. When you are sure of the arrangement, glue or tape the pictures to a piece of paper. Don’t forget to leave a space for your sketch of Cousin Al. 5. Answer the discussion questions. 1234 Atomic Avenue Periodic, Texas Dear Cousin Beryl: What’s happening? I’m writing because Grandmother has a favor to ask of you. She says you are the most artistic and most organized member of the family. I think she always did like you best. Anyway, here’s the deal. Grandmother has collected pictures of all the grand kids. She wants you to make a collage by arranging the photos in rows from the smallest cousin to the largest cousin. She also said to tell you that kids who belong to the same family are dressed alike. Grandmother would like the members of each family arranged in their own vertical column. I hope you will understand what she means when you see the pictures. By the way, I said she has a picture of everyone. Actually, she’s missing the picture of cousin Al. Grandmother says that, with your talent, you should be able to sketch what Al looks like because his features are similar to the cousins who surround him. Got to go! In a way, I’m glad she does like you best! Good luck and don’t disappoint her! Best Wishes, Cousin Fluora “WHERE’S AL?” Discussion Questions 1. In this activity, all parts of the cousins represent properties or particles of an atom. Next to each part, write the term from an atom that represents each part. Some parts may have more than one term. Word bank: atomic mass electrons group number reactivity energy levels group members valence electrons period number _______________________________ number of arms _______________________________ number of fingers _______________________________ number of hairs on head _______________________________ size of body _______________________________ smile or lack of smile _______________________________ clothing type 2. Why are some cousins smiling while others are frowning? (HINT: think about the valence electrons) __________________________________________________________ __________________________________________________________ 3. Write the name of the element represented by each cousin beneath their picture by comparing the pictures to the Periodic Table. 4. Write three generalizations about the Periodic Table. The first two generalizations are started for you. a. The Periodic Table is arranged__________________________________ _____________________________________________________________ b. Groups on the Periodic Table ___________________________________ _____________________________________________________________ c. __________________________________________________________ _____________________________________________________________ _____________________________________________________________ 5. Think about how you could show each cousin’s unique identity as well as the electric charge. Draw this feature on your Al sketch and one other cousin. Cousins Page Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω Ω