Supplementary Information (doc 82K)
advertisement

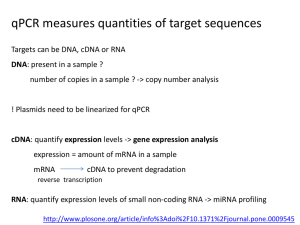
Supplementary Information Supplementary Methods ......................................................................................... 2 References ............................................................................................................... 5 Table S1. Overview of sequencing samples .......................................................... 6 Table S2. Genome coverage of each sequencing library by regions .................. 6 Table S3. List of ADAR1-binding transcripts identified in the study ................... 6 Table S4. List of primers used in this study .......................................................... 6 Legends to Supplementary Figures ....................................................................... 7 1 Supplementary Methods Reagents and antibodies All chemicals were purchased from Sigma (St. Louis, MO), except where otherwise indicated. Anti-cleaved caspase 3 (polyclonal), anti-α-tubulin (polyclonal) and anti-DNM1 (monoclonal) antibodies were from Cell Signaling Technology (Danvers, MA, USA). Monoclonal antibodies against MyoG, MyoD, MHC, ADAR1, ADAR2, and GAPDH, as well as polyclonal antibodies against PKR and phosphorylated form of PKR were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-ADAR1 rabbit polyclonal antibody was purchased from Abnova (Taiwan). ARHGAP26 and XIAP rabbit polyclonal antibodies were purchased from Abcam (Cambridge, MA, USA). Anti-Anxa4 antibody was obtained from EnoGene Biotech (New York, NY, USA). Secondary antibodies used in the western blot assays were from Jackson Laboratories (West Grove, PA, USA), whereas those used in immunofluorescence analysis were obtained from Invitrogen. RNA extraction and quantitative reverse transcription (RT)-PCR Total RNA from cells was isolated using the TRIzol reagent (Invitrogen) according to manufacturer’s instructions. cDNA and microRNA cDNA were synthesized by MMLV reverse transcriptase (Invitrogen) using random hexamers and microRNA RT-loop primers, respectively. Sequences for the microRNA RT-loop primers and primers for real-time PCR assays are listed in Supplementary Table S4. Quantitative determination of the cDNA levels was done by real-time PCR using the Bio-Rad iQ5 Gradient Real Time SYBR-Green PCR system. Levels of cDNA were normalized to the GAPDH values of the respective samples, and microRNA cDNA was normalized to U6 control. All results represent the mean ± SD of at least three independent experiments. Cell lysate preparation Cells were harvested and washed twice in PBS. Whole cell extracts were prepared using WCE buffer [20 mM HEPES, pH 7.4, 0.2 M NaCl, 0.5% Triton X-100, 5% glycerol, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 2 mM Na3VO4, 1 mM NaF, 1 mM DTT, and cocktail protease inhibitor (Roche)]. After 30-min incubation on ice, whole cell extracts were collected by centrifugation (12000 g, 20 min). Bromodeoxyuridine (BrdU) ELISA cell proliferation assay The proliferative capacity of cells was measured using a BrdU ELISA cell proliferation assay (Millipore) according to the manufacturer’s instructions. Cultured cells were incubated with BrdU labelling solution for 12 hrs and incubated with monoclonal antibody against BrdU for 1 hr. The BrdU absorbance was measured directly using a spectrophotometric microplate reader at a test wavelength of 450 nm and a reference wavelength of 540 nm. This provided a measure of the degree of proliferation of cells, which we termed the proliferation index. Each sample was cultured in quadruplicate. Western blot analysis and immunoprecipitation 2 Western blot analysis was performed after electrophoretic separation of polypeptides by SDS-PAGE and transfer to Immobilon-P/PVDF membrane (Millipore). Blots were probed with the indicated primary and appropriate secondary antibodies. Immuno-bands were subsequently detected by the enhanced chemiluminescence reaction (ECL) (PerkinElmer). All immunoprecipitations were performed with equal amounts of cell extract protein incubated with the indicated antibodies (2.5 μg) at 4°C for overnight with rotation. The immunocomplexes were captured with protein G-sepharose (30 μl) (Millipore) for 2 hrs at 4°C with rotation. The protein G-antigen-antibody complexes were washed six times with the WCE buffer, and boiled in 2× urea sample buffer dye for subsequent PAGE and immunoblotting analysis as described above. Sequence alignments and microRNA target prediction The potential microRNA target sites within the 3’UTR of ADAR1 were predicted using web-based bioinformatics database TargetScan (http://www.targetscan.org). Synthetic microRNA mimics For ectopic expression of miRNA mimics and antagomers, C2C12 myoblasts or HeLa cells were transiently transfected with the indicated synthetic RNA oligonucleotides and corresponding control (Thermo Fisher Scientific) using Lipofectamine 2000 (Invitrogen). Overexpression of miR-206 was also performed by a plasmid-based system (pcDNA6.2) harboring coding sequence for either miR-206 or LacZ and subsequent transfection using Lipofectamine 2000 (Invitrogen). Promoter and 3’UTR reporter constructs, mutagenesis, and luciferase reporter assay To generate 3’UTR reporter, a 2,268-bp PCR fragment corresponding to mouse ADAR1 3’UTR was subcloned into the luciferase vector pMIR-REPORT (Ambion). Site-directed mutagenesis was employed to generate a mutant version that harbor altered miR-1/206 target site (predicted seed complement GGAATGT mutated to GGTTAGT; Mt). For 3’UTR reporter assay, C2C12 cells were seeded in 12-well plate before transfection. Co-transfections were done with the reporter constructs and the indicated synthetic miRNA using Lipofectamine 2000 (Invitrogen). After 48 hr incubation, cells were lysed in Reporter Lysis 5× Buffer (Promega) and centrifugated to remove cell debris. Luciferase activity was measured by a Dual-Luciferase Reporter Assay System (Promega). The co-expressed β-gal was used as loading control. Over-expression and ectopic expression constructs Plasmid construction was based on the pFLAG-CMV (Sigma), pCMV-SPORT6 (for C-terminal FLAG tagging), and pIRES2-EGFP system (Clontech), which is also driven by the CMV promoter. For over-expression during initial differentiation, cDNAs encoding full-length mouse ADAR1 p150 and p110 were expressed from pFLAG-CMV. Mutations were created by PCR-based mutagenesis in the dsRBDs 3 (K618/619/622/726/727/730A) or the catalytic domain (E613A for p110 or E861A for p150) of ADAR1, and corresponding constructs were generated as described previously (Lai et al., 1995; Valente and Nishikura, 2007). PCR mutagenesis was done using HiFi Hotstart DNA Polymerase (KAPA Biosystems) with mutagenic oligonucleotides creating specific codon changes (mutations in bold): dsRBD-EAA2, 5’-GTGAGCGCCCCCAGCGAGGCGGTAGCAGCGCAGATGG-3’; dsRBD-EAA3, 5’-CGTGTGTGCACACAGCGAGGCACAGGGCGCGCA-3’; and CT-EA, 5’-GTCAATGACTGCCATGCCGCAATCATCTCCCGGAGGGGCTTC-3’. For ectopic expression of ADAR1 during late differentiation, we modified the pFLAG-CMV and pIRES2-EGFP vector by replacing the CMV promoter with muscle-specific MCK upstream regulatory sequence (Takeshita et al., 2007). The mouse MCK promoter (nucleotides -1256 to 0 relative to transcript start site) was PCR-amplified from genomic DNA isolated from C2C12 cells. The CMV promoters in pFLAG-CMV and pIRES-EGFP were released by restriction digestion subsequently replaced with the MCK promoter amplicon, products of which were respectively termed pFLAG-MCK and pIRES2-MCK. Indirect immunofluorescence and confocal microscopy C2C12 cells were fixed in 4% paraformaldehyde and permeabilized in 0.5% Triton X-100. Cells were then blocked in blocking buffer (1% BSA in PBS) and probed with the indicated primary antibodies and species-specific secondary antibodies (Alexa 488- or 594-conjugated anti-mouse or anti-rabbit IgG). Cell nuclei were counter-stained with DAPI (Sigma); F-actin was stained with phalloidin. Images were captured on a Zeiss LSM 510 Meta confocal laser-scanning microscope (Carl-Zeiss, Feldbach, Switzerland), using a 63×/NA 1.4 oil immersion objective lens. The fusion index was calculated as the ratio of nuclei number in myocytes versus the total number of nuclei. Each data point was generated from at least 1,500 randomly chosen MHC-positive cells or myotubes. Nuclear run-on assay The nuclear run-on assay was carried as described previously (Hsieh et al., 2011). Cells were suspended in lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, Digitonin). After 10-min incubation, nuclei were collected and then washed with lysis buffer devoid of Digitonin. To perform run-on reactions, aliquots of nuclei were mixed with 100 µl of 2× reaction buffer (20 mM Tris-HCl, pH 8.0, 5 mM MgCl2, 200 mM KCl, 4 mM dithiothreitol, 1 mM each of ATP, CTP, and GTP, 200 mM sucrose and 20% glycerol) and biotin-16-UTP (Roche) in a final volume of 200 µl at 29°C for 30 min. A total of 60 U of RNase-free DNaseI (Fermentas; Burlington, Ontario, Canada) and 6 µl 250 mM CaCl2 were added, and the reaction mixture was incubated for an additional 10 min at 37°C. The nuclear run-on RNA and total RNA were then digested with DNase I (Ambion) to further remove contaminating genomic DNA. Biotinylated RNA was purified by Dynabeads M-280 (Invitrogen), a magnetic bead covalently linked to streptavidin. Beads were washed once with 500 µl 2× 4 SSC-15% formamide for 10 min and twice with 1 ml 2× SSC for 5 min each. Random hexamer-primed cDNA was synthesized using 10 µl biotinylated RNA and 500 ng total RNA, and subsequently subjected to reverse transcription-quantitative PCR to assay for RNA transcription rate. To ensure the efficiency of the reverse transcription, the intensities of PCR products were normalized to those of U6 snRNA. Nuclear and cytoplasmic fractionation Cells were washed once with PBS and lysed with the nuclear fractionation buffer containing 10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 0.2% NP-40, 3 mM MgCl2, protease inhibitor (Roche), 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate and 1 mM sodium fluoride for 10 min at 4°C. Cells were centrifuged at 800 g for 5 min at 4°C. The supernatant was removed and used as a cytoplasmic fraction. The pellet was washed once with nuclear fractionation buffer and centrifuged again at 800 g for 10 min at 4°C. The pellet was used as a nuclear fraction. Cytoplasmic and nuclear fractions were controlled using GAPDH and Lamin B as respective markers. References Hsieh, C.L., Lin, C.L., Liu, H., Chang, Y.J., Shih, C.J., Zhong, C.Z., Lee, S.C., and Tan, B.C. (2011). WDHD1 modulates the post-transcriptional step of the centromeric silencing pathway. Nucleic Acids Res 39, 4048-4062. Lai, F., Drakas, R., and Nishikura, K. (1995). Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J Biol Chem 270, 17098-17105. Takeshita, F., Takase, K., Tozuka, M., Saha, S., Okuda, K., Ishii, N., and Sasaki, S. (2007). Muscle creatine kinase/SV40 hybrid promoter for muscle-targeted long-term transgene expression. Int J Mol Med 19, 309-315. Valente, L., and Nishikura, K. (2007). RNA binding-independent dimerization of adenosine deaminases acting on RNA and dominant negative effects of nonfunctional subunits on dimer functions. J Biol Chem 282, 16054-16061. 5 Table S1. Overview of sequencing samples (Please see separate MS Excel file.) Table S2. Genome coverage of each sequencing library by regions (Please see separate MS Excel file.) Table S3. List of ADAR1-binding transcripts identified in the study (Please see separate MS Excel file.) Table S4. List of primers used in this study (Please see separate MS Excel file.) 6 Legends to Supplementary Figures Figure S1. C2C12 cells were transfected with plasmid construct encoding C-terminally FLAG-tagged wild-type p150. The empty vector was used as a control (ctrl). Cells were analyzed for expression levels of the indicated proteins. Expression of the FLAG-p110 form from this construct, denoted by black arrowheads, may be an indication of alternative translation via internal start codon. Figure S2. Comparison of the proliferation status of the ADAR1 knockdown (left) and over-expression (right) cultures in the C2C12 differentiation experiments (Figure 1, c & e). Proliferation status was determined as described in the Supplementary Methods based on the relative proportion of replicating cells. Figure S3. Relative expression levels of miR-1 and miR-206 in differentiating C2C12 (0, 24, 48, 72, and 96 hrs post-DM culture), as determined by real-time RT–PCR. Figure S4. Genotyping results for the Adar1 transgenic mice (related to Figure 3d). (a) Genotyping analysis of genomic DNA isolated from the amnion of 13.5-day old embryos. Samples were analyzed by PCR detecting ADAR1 wild-type (Wt) and Transgenic (Tg) alleles. DNA size marker (M), genotyping positive control (ctrl; transgene construct as the template), and no-template controls (NTC) are as indicated. Lengths of the product amplicons are shown on the bottom. (b) WISH on wild-type (Wt) and transgenic (Tg; with MCK-Adar1 p110) embryos of identical somite numbers at embryonic stage E13.5. Probe corresponding to Adar1 was used to monitor marker expression and somite structure, as indicated. Lateral view of whole embryo is shown on the left of each panel, and magnification of boxed thoracic regions on the right. The expression domain of Adar1 in interlimb somoites is denoted by brackets, (c) Quantitative assessment of MyoD WISH signals shown in Figure 3d. Relative extent of MyoD expression in interlimb somites, in terms of domain length, was determined for Tg versus Wt mice. Numbers on the x-axis denote arbitrary somite regions, in the anterior-to-posterior organization. Figure S5. RIP-Seq-based identification of ADAR1-binding targets (related to Figure 4). (a) Overrepresentated GeneGo Maps in the edited gene sets, as revealed by pathway analysis using MetaCore. (b) Read density plots for additional targets showing representation in the four sequencing libraries. Corresponding gene structure is depicted on top of each plot. X 7 axis denotes relative position of the gene whereas y axis corresponds to number of distinct reads. (c) Preponderance of differentially bound gene transcripts in distinct biological processes, as revealed by analysis using MetaCore. The top GeneGo process networks, based on statistical significance of enrichment, are shown for DM-0 h targets. (d) Additional candidate targets of ADAR1 were confirmed by RIP-qRT-PCR. Analyses for RIP enrichment and stage-dependent enrichment of ADAR1 transcript binding were done as in Figure 4, d & e, respectively. (e) Differentiation-associated mRNA expression profiling of selected ADAR1-targeted candidates. C2C12 cells were cultured in GM or in DM for the indicated time lengths. RNA was isolated from these cells and subjected to real-time RT-PCR with primers specific to the indicated genes. The bar graphs show normalized values with GM cells represented as 1. (f) Effect of ADAR1 down-regulation on the mRNA levels of selected candidate targets, as shown. C2C12 cells were transiently transfected with siRNAs targeting Adar1 or control siRNA (ctrl; targeting GFP), and harvested at DM-48 h for qRT-PCR analysis. Figure S6. Sanger sequencing analysis of target gene transcripts. Sanger sequencing analysis was performed for cDNA isolated from C2C12 cells in DM-0 hr vs. DM-72 hr culture. Candidate editing sites in DNM1 and DNM2 genes are highlighted by grey shades and genomic coordinates (mm9). 8