XRF: X-Ray Fluorescence Spectroscopy Analysis
advertisement

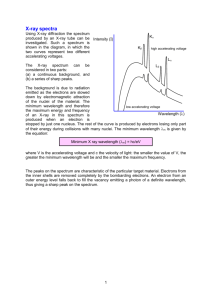
X-Ray Fluorescence (XRF) Becoming the most widely used method for elemental analysis of solids ADVANTAGES AND DISADVANTAGES Advantages of X-Ray Spectrometry * Simple spectra * Spectral positions are almost independent of the chemical state of the analyte * Minimal sample preparation * It is non-destructive * Applicable over a wide range of concentrations * Good precision and accuracy Disadvantages of X-Ray Spectrometry * X-ray penetration of the sample is limited to the top 0.01 - 0.1 mm layer * Light elements (below 22Ti) have very limited sensitivity although C is possible on new instruments * Inter element (MATRIX) effects may be substantial and require computer correction * Limits of detection are only modest * Instrumentation is fairly expensive NOMENCLATURE Simplified spectral lines observed in x-ray spectra (each energy shell actually comprises several energy levels, thus transitions are more numerous than shown). PRINCIPLES OF X-RAY FLUORESCENCE X-Ray Excitation Electron Excitation AUGER ELECTRON EMISSION (internal photoionisation) The Auger effect is more common in elements of low Z because their atomic electrons are more loosely bound and their characteristic Xrays more readily absorbed. X-RAY FLUORESCENCE YIELD The yield of X-ray photons is reduced by the Auger effect. The fluorescence yield ( ) is the ratio of X-ray photons emitted from a given shell to the number of vacancies created in that shell. Since production of Auger electrons is the only other competing reaction the ratio of Auger electrons to vacancies must be 1- . X-RAY ABSORPTION AND SCATTERING IN CONDENSED PHASES The probability of X-ray absorption as a function of path length through the sample is given by Beer's law for X-rays: I/I0 = exp(-µm d) where I/I0 is the fraction of X-rays transmitted through a thickness d of a material of density . The parameter µm is the mass absorption coefficient which is a function of the atomic number Z and the energy (wavelength) of the X-ray. The mass absorption coefficient for a complex sample is the weighted average of the coefficients for the constituent atoms. X-RAY MASS ABSORPTION COEFFICIENTS A plot of mass absorption coefficient vs energy of the X-ray photon for 82Pb. Abrupt changes are observed corresponding to absorption edges for K, L and M electrons. At the energy (wavelength) of the edge, the photons first become sufficiently energetic to eject K, L and M photoelectrons. X-RAY SOURCES The X-ray tube is energised by a high-voltage power supply with an output of 0.5 to 50 kV. The head of the vacuum tube consists of a target (anode), which is often made of tungsten and chromium. As accelerated electrons strike the target, X-rays are emitted. The tube also forms the X-rays into a beam through a Beryllium window. In the target the 74W is used to excite L and K lines of higher Z atoms and the 24Cr is used to excite atoms of 22Ti and below. Spectral Output X-ray spectrum produced by electron bombardment of a tungsten target: Continuum Spectrum: The continuum results from deceleration of electrons by the atoms in the target. The German term "bremsstrahlung" means "braking radiation." Characteristic Spectra: Electron bombardment also produces characteristic peaks K and K provided the accelerating potentials are sufficiently high. DETECTORS X-ray photons (as well as other energetic particles) can be measured using the following types of detectors: * gas-filled detectors register a current pulse from the collection of electron-ion pairs formed; * a semiconductor detector register a current pulse from the formation of electron-hole pairs; * a scintillation detector counts light pulses created when an X-rays passes through a phosphor; * a photographic plate. Gas-Proportional Counters A gas-proportional counter is filled with P90 gas (90% argon, 10% methane). X-rays ionise the gas, leaving electrons that migrate to the anode and positive ions that move to the case. Proportional counters use gas amplification: the detector voltage is raised to 500 to 700 V so that the primary electrons and ions, first formed, are accelerated to produce secondary electrons and ions when they collide with gas atoms. This yields a greatly increased signal which is, nevertheless, proportional to the energy of the original x-ray. Solid-State Detectors When an electron enters the crystal it ejects a high-energy photoelectron which ultimately dissipates its energy in multiple interactions which promote valence band electrons to the conduction band, leaving holes in the valence band. The electron-hole pairs are then collected by biasing the detector at -1000 V, giving rise to a current pulse for each x-ray entering the detector. Charge collection is much more efficient than in a gas. Lithium-Drifted Si(Li) Detectors A lithium-drifted Si(Li) detector is manufactured from high-purity p-type silica. However, p-type silica of sufficiently high purity is difficult to fabricate. Most Si crystals contain extrinsic holes, caused by impurities, which allow significant "leakage" of current at the required bias voltage. In order to compensate for these extrinsic holes, lithium, an n-type dopant, is diffused into the material at 350 - 450ºC under an electrical gradient. The lithium atoms compensate for the extrinsic charge-carriers in the p-type silicon and provide a wide "intrinsic" region of high resistance. Si(Li) detectors are operated at 77 K with a liquid N2 cryostat to prevent further diffusion and to reduce the level of random noise due to the thermal motion of charge carriers. WAVELENGTH DISPERSIVE XRF SPECTROSCOPY Instrumentation In wavelength dispersive spectrometers, the several x-ray lines emitted from the sample are dispersed spatially by crystal diffraction on the basis of wavelength. The detector then receives only one wavelength at a time. The crystal and detector are made to synchronously rotate through angles of θ and 2θ respectively. Bragg Diffraction Wavelength dispersive X-ray spectrometers function by separating the X-rays of interest using diffraction from a crystal. This follows from the Bragg equation: n = 2 d sin( ) where n is the diffraction order, d is the interplanar spacing of the atomic layer and the angle of incidence. Crystals Crystal Primary Range LiF 0.025 - 0.272 nm Si 0.055 - 0.598 nm pentaerythritol 0.076 - 0.834 nm CaSO4.2H2O 0.132 - 1.45 nm KAP* 0.232 - 2.54 nm lead stearates 6 - 15 nm is *Potassium hydrogen phthalate ENERGY DISPERSIVE XRF SPECTROSCOPY The primary X-ray beam excites several spectral lines from the sample. In energy dispersive XRF all wavelengths enter the detector at once. The detector registers an electric current having a height proportional to the photon energy. These pulses are then separated electronically, using a pulse analyser. WAVELENGTH AND ENERGY DISPERSION COMPARED Advantages of Energy Dispersion: * simplicity of instrumentation - no moving parts * simultaneous accumulation of the entire Xray spectrum * qualitative analysis can be performed in 30 s, or so * a range of alternative excitation sources can be used in place of high-power x-ray tubes with their large, heavy, expensive and powerconsuming supplies * alternative sources include, low power x-ray tubes, secondary monochromatic radiators, radioisotopes and ion beams. Advantages of Wavelength Dispersion: * resolution is better at wavelengths longer than 0.08 nm * higher individual intensities can be measured because only a small portion of the spectrum is admitted to the detector * with multichannel analysers sensitivity for weak lines in the presence of strong lines is limited because the strongest line determines the counting time * lower detection limits are possible SAMPLE PREPARATION Reproducible sample preparation methods are essential. Samples must be in a form that are similar to available standards in terms of matrix, density and particle size. * Solids, generally solids must be polished as surface roughness may give erratic results. * Powders and pellets, powdered samples are often pressed into pellets, suspensions may also be analysed * Fusions, with potassium pyrophosphate (K2P2O7) or a tetraborate (Na2B4O7 or Li2B4O7) present a homogenised sample which can often be analysed directly * Liquids and solutions, a x-ray transparent cover and sample cup must be provided to prevent volatility under vacuum conditions * support media, such as filter paper, millipore filters, ion-exchange membranes MATRIX EFFECTS Types of Matrix Effects In XRF absorption-enhancement effects arise from the following phenomena: 1. The matrix absorbs primary x-rays (primary-absorption effect); it may have a larger or smaller absorption coefficient than the analyte for primary source x-rays 2. The matrix absorbs the secondary analyte xrays (secondary-absorption effect); it may have a larger or smaller absorption coefficient for the analyte-line radiation 3. The matrix elements emit their own characteristic lines, which may lie on the short wavelength side of the analyte absorption edge, thereby exciting the analyte to emit additional radiation to that excited by the primary source of X-rays alone (enhancement) Absorption-Enhancement Effects Absorption-enhancement effects can be positive or negative on the basis of their effect upon analyte intensity. In the positive absorption effect, the matrix has a smaller absorption coefficient than the analyte for the primary and analyte-line radiation, and the analyte-line radiation is higher than would be predicted. In the negative absorption effect, the matrix has a larger absorption coefficient than the analyte, and the analyte-line intensity is lower than expected. In the enhancement effect, one or more spectral lines of the matrix elements excite analyte-line emission). This enhancement may take two forms: direct enhancement ( C both excite A) B and the third element and enhancement ( excites C excites B which in turn A). QUANTITATIVE ANALYSIS 1. Calibration-Standard Methods. The analyteline intensity from samples is compared with that from standards having the same form as the samples and, nearly as possible, the same matrix. 2. Internal Standardisation. The calibrationstandard method is improved by quantitative addition to all samples of an internal standard element having excitation, absorption and enhancement characteristics similar to those of the analyte in the particular matrix. The calibration function involves measuring the intensity ratio of the analyte and internal standard lines. 3. Matrix-Dilution Methods. The matrix of all samples is diluted to a composition such that the effect of the matrix is determined by the diluent rather than the matrix. 4. Thin-Film Methods. The samples are made so thin that absorption-enhancement effects substantially disappear. 5. Standard Addition and Dilution Methods. The analyte concentration is altered quantitatively in the sample itself. The sample is subjected to one or more quantitative incremental concentrations or dilutions of the analyte. The intensity of the analyte lines is measured for effectively the same matrix in each case. 6. Mathematical Corrections. Absorptionenhancement effects are corrected mathematically by the use of influence coefficients for each element present (these are derived experimentally from reference samples). The basic approach is that the XRF intensity at a particular wavelength will in some way be affected by each element in the sample.