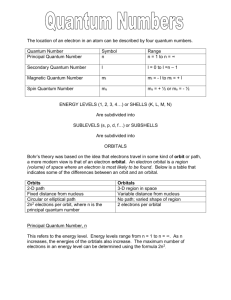
These are sample problems from Chapter 7
advertisement

These are sample problems from Chapter 7. 1. What is the wavelength of radiation that has a frequency of 2.10 × 1014 s–1? A. B. C. D. E. 2. Calculate the frequency of visible light having a wavelength of 486.1 nm. A. B. C. D. E. 3. 6.46 × 10–16 J 6.46 × 10–25 J 2.46 × 10–4 J 12.4 kJ 246 kJ Complete this sentence: Atoms emit visible and ultraviolet light A. B. C. D. E. 5. 2.06 × 1014/s 2.06 × 106/s 6.17 × 1014/s 1.20 × 10–15/s 4.86 × 10–7/s What is the energy in joules of a mole of photons associated with visible light of wavelength 486.1 nm? A. B. C. D. E. 4. 6.30 × 1022 m 7.00 × 102 nm 7.00 × 105 m 1.43 × 10–6 m 3.00 × 108 m as electrons jump from lower energy levels to higher levels. as the atoms condense from a gas to a liquid. as electrons jump from higher energy levels to lower levels. as they are heated and the solid melts to form a liquid. as the electrons move about the atom within an orbit. Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 6 to the n = 3 principal energy level. Recall En = -2.18 × 10–18 J(1/n2) A. B. C. D. E. 1.64 × 1015/s 9.13 × 1013/s 3.65 × 1014/s 1.82 × 10–19/s 2.70 × 1014/s Page 1 6. Which one of the following sets of quantum numbers is not possible? n A. B. C. D. E. 7. 4 3 3 2 2 l 3 0 0 1 0 -2 1 0 1 0 A. B. C. D. E. l 4 3 3 4 2 3 2 0 1 0 l=+3 A. 0 4 3 4 3 4 l 0 1 1 1 2 ml= -2 ms = + 1/2 C. 2 D. 6 E. 10 ml 0 0 0 1 1 ms - 1/2 - 1/2 + 1/2 + 1/2 + 1/2 B. f orbital. C. g orbital. D. p orbital. E. s orbital. D. 5 E. 7 The number of orbitals in a d subshell is A. 1 B. 2 C. 3 The maximum number of electrons that can occupy an energy level described by the principal quantum number, n, is A. n 13. + 1/2 - 1/2 + 1/2 - 1/2 + 1/2 Electrons in an orbital with l = 3 are in a/an A. d orbital. 12. -2 -3 0 1 0 ms A possible set of quantum numbers for the last electron added to complete an atom of gallium Ga in its ground state is A. B. C. D. E. 11. ml B. 1 n 10. + 1/2 - 1/2 + 1/2 - 1/2 + 1/2 The maximum number of electrons in a atom that have the following set of quantum numbers is n=4 9. ms Which one of the following sets of quantum numbers is not possible? n 8. ml B. n + 1 C. 2n D. 2n2 E. n2 How many orbitals are allowed in a subshell if the angular momentum quantum number for electrons in that subshell is 3? A. 1 B. 3 C. 5 D. 7 Page 2 E. 9 14. "No two electrons in an atom can have the same four quantum numbers" is a statement called A. B. C. D. E. 15. the Pauli exclusion principle. Bohr's equation. Hund's rule. De Broglie's relation. Dalton's atomic theory. The orbital diagram for a ground state nitrogen atom is 1s A. B. C. D. 16. The orbital diagram for a ground state oxygen atom is A. B. C. D. E. 2s The orbital diagram for a ground state carbon atom is A. B. C. D. 2s 2p How many unpaired electrons does an atom of sulfur have? A. 0 19. 2p 1s 18. 2p 1s 17. 2s B. 1 C. 2 D. 3 E. 4 Which element has the following electron configuration? [Kr]5s24d105p3 A. Sn 20. B. Sb C. Pb D. Bi E. Te Which element has the following electron configuration? [Kr]5s24d105p2 A. Sn B. Sb C. Pb Page 3 D. Ge E. Te 21. The electron configuration of a ground state Co atom is A. B. C. D. E. 22. The electron configuration of a copper atom is A. B. C. D. E. 23. 0, diamagnetic 2, diamagnetic 3, paramagnetic 5, paramagnetic 7, paramagnetic Transition metal elements have atoms or ions with partially filled A. B. C. D. E. 26. [Kr]5s24p64d5 [Ar]4s23d104p1 [Ar]4s24p63d5 [Kr]5s25p64d5 [Kr]5s24d105p1 An atom of manganese has ___ unpaired electrons and is _____. A. B. C. D. E. 25. [Ar]4s24d4 [Ar]4s24p63d3 [Ar]4s23d7 [Ar]3d9 [Ar]4s13d10 The ground state electron configuration for an atom of indium is A. B. C. D. E. 24. [Ar]4s23d7 1s22s22p63s23d9 [Ne]3s23d7 [Ar]4s13d5 [Ar]4s24d7 s subshells. p subshells. d subshells. f subshells. g subshells. Lanthanide or rare earth elements have atoms or ions with partially filled A. B. C. D. E. s subshells. p subshells. d subshells. f subshells. g subshells. Page 4 27. Which choice lists two elements with electron configurations that are well-known exceptions to the Aufbau principle? A. B. C. D. E. Cu and C Cr and Cu Cs and Cl Rb and Co Fe and Co 28. The Bohr model for the hydrogen atom found its best support from experimental work on the photoelectric effect. 29. An electron in a 3p orbital could have a value of 2 for its angular momentum quantum number 1. 30. A neon atom in its ground state will be diamagnetic. 31. Each shell (principal energy level) of quantum number n contains n subshells. 32. For all atoms of the same element, the 2s orbital is larger than the 1s orbital. 33. According to de Broglie's equation, the wavelength associated with the motion of a particle increases as the particle mass decreases. 34. The frequency of the emitted light from a cesium atom is an intensive property. Page 5 Answer Key for Test "Sample_CH7.tst", 11/25/2003 No. in Q-Bank 7-2 7-3 7-6 7-9 7-12 7-19 7-20 7-21 7-22 7-24 7-25 7-26 7-27 7-28 7-29 7-30 7-31 7-32 7-34 7-35 7-36 7-38 7-39 7-43 7-46 7-47 7-48 7-71 7-72 7-73 7-74 7-75 7-76 7-77 No. on Test 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Correct Answer D C E C E B B B C B D D D A A D D C B A A E E D C D B F F T T T T T Page 6