Volume, Mass and Density
advertisement
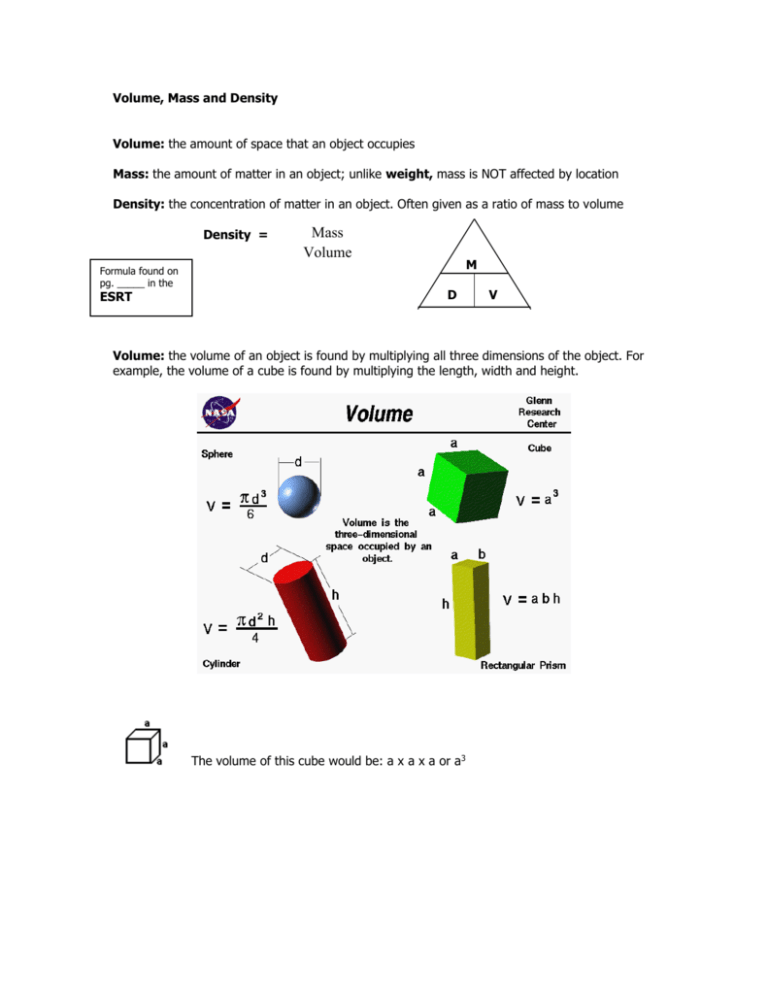
Volume, Mass and Density Volume: the amount of space that an object occupies Mass: the amount of matter in an object; unlike weight, mass is NOT affected by location Density: the concentration of matter in an object. Often given as a ratio of mass to volume Density = Formula found on pg. _____ in the ESRT Mass Volume M D V Volume: the volume of an object is found by multiplying all three dimensions of the object. For example, the volume of a cube is found by multiplying the length, width and height. The volume of this cube would be: a x a x a or a3 (Example A) What is the volume of this cube if all sides are 3cm? 3cm x 3cm x 3cm = 27cm3 DON’T FORGET: cm to the third power! (Example B) What is the volume of this one if all sides are 1.5cm? 1.5cm x 1.5cm x 1.5cm = 3.375cm3 Volume units are always given in some distance cubed… (ft3 , m3 , cm3 ) *Volume units are always labeled in some distance cubed… (cm 3, ft3, m3) Most often in this class we will use cm3. Mass: How much matter… does it matter?? Mass is the amount of matter in a substance or object. If there are a lot of molecules of matter OR if the molecules are very large molecules, there will be a high mass. Instruments to measure density: Density of a liquid: ____________________________ Density of a solid: ________________________ and ________________________ (Example C) In the diagram to the left, the mass of object x=80 grams. The volume can be determined by the change in the fluid level. What is the density of object X? _______________ Mass = 80 grams Fluid level changes from 40 to 65 cm3, so volume = 65-40… which = 25 cm3 Density = 80 g / 25 cm3 = 3.2 g/cm3 Total Mass = 6 g Total Mass = 24 g Total Volume = 1 cm3 Total volume = 4 cm3 Density = 6 g/cm3 Density = 6 g/cm3 What is the relationship between size and density?? As size increases or decreases, DENSITY REMAINS THE SAME! As a rule, things that are MORE dense will sink or fall to the bottom of things that are LESS dense (think of oil and water). My friend is a comic book freak. He thinks he wants to fly like superman, but in order to do that, he would have to have a density LESS than air. What are two things that he could do to decrease his density? THINK ABOUT IT! (Hint – look at the density equation. Play with some numbers.) Notice that if you DECREASE the mass of something or INCREASE the volume, the density will be less… D=M/V D = 3g / 6cm3 D = 6g / 6cm3 D = 1g/cm3 D = 0.5 If we DECREASE the mass… Or if we INCREASE the volume… g/cm3 D = 6g / 12cm3 D = 0.5 g/cm3 *** If you cut a piece of anything in half, you cut the mass and the volume in half as well, so a certain material ALWAYS HAS THE SAME DENSITY. THE DENSITY OF ANY MATERIAL NEVER CHANGES









