Mole calculations
advertisement

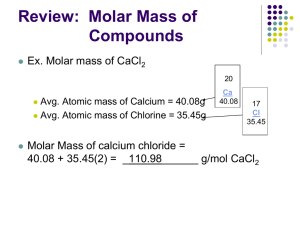
Higher Chemistry What mass of oxygen contains 12000000000000 00000000000000 0 atoms? Oh no, I can't take any more! Mole Calculations Moles and Number 1 mole of any substance contains the same number of atoms/molecules/ions as there are atoms in a 12g sample of carbon -12 12g of carbon-12 contains a constant number of atoms. This number is known as the Avogadro constant (L) and is equal to 6.02 x 1023. 1 mole of carbon-12 contains 6.02 x 1023 atoms. Therefore, 1 mole of any substance contains 6.02 x 1023 atoms/molecules/ions. For example, 1 mole of aluminium contains 6.02 x 1023 Al atoms 1 mole of ammonia contains 6.02 x 1023 NH3 molecules 1 mole of iron ions contains 6.02 x 1023 Fe ions Atoms Calculate the number of atoms in 2 moles of silicon. Number of Atoms = = = moles x atoms in 1 mole 2 x 6.02 x 1023 1.20 x 1024 atoms Calculate the number of atoms in: (a) 3 moles of phosphorus (b) 0.5 moles of sodium Answers (a) 1.81 x 1024 atoms (b) 3.01 x 1023 atoms Molecules Calculate the number of molecules in 6 moles of methane Number of Molecules = = = moles x molecules in 1 mole 6 x 6.02 x 1023 molecules 3.61 x 1024 molecules Calculate the number of molecules in: (a) 0.37 moles of sulphur dioxide (b) 3.4 moles of hydrogen chloride Answers (a) 2.23 x 1023 molecules (b) 2.05 x 1024 molecules Ions Ionic compounds do not contain atoms or molecules. Instead, we talk about ‘formula units’ The formula unit (f.u.) is the same as the chemical formula Calculate the number of formula units in 2 moles of magnesium bromide [MgBr2] Number of Formula Units = = = moles x f.u. in 1 mole 2 x 6.02 x 1023 f.u. 1.20 x 1024 f.u. Calculate the number of ions in 0.25 moles of potassium sulphate [K2SO4] 1 formula unit = 6.02 x 1023 formula units = 3 ions (K + K + SO4) 3 x 6.02 x 1023 of ions Number of ions moles x ions in 1 mole 0.25 x 3 x 6.02 x 1023 4.52 x 1023 ions = = = Calculate the number of: (a) copper ions in 0.4 moles of copper(II)oxide [CuO] (b) nitrate ions in 3.2 moles of calcium nitrate [Ca(NO3)2] Answers: (a) 2.41 x 1023 Cu ions (b) 3.85 x 1024 NO3 ions Number to Moles Calculate the number of moles of lithium containing 3.01 x 1022 atoms Number of moles = atoms in question atoms in 1 mole = 3.01 x 1022 6.02 x 1023 = 0.05 moles Calculate the number of moles of: (a) barium iodide which contains 1.82 x 1023 barium ions (b) water containing 4.52 x 1022 atoms of hydrogen Answers (a) 0.30 moles (b) 0.038 moles Mass to Molecules or Formula Units How many molecules are in 9.13g of hydrogen chloride? Number of moles of hydrogen chloride Number of molecules = mass mass 1 mole = 9.13 36.5 = 0.25 moles = moles x molecules in 1 mole = 0.25 x 6.02 x 1023 molecules = 1.51 x 1023 molecules How many molecules are in: (a) 56g of methane? (b) 3.2g of sulphur dioxide? (c) 1.5kg of nitrogen? Answers (a) 2.11 x 1024 molecules (b) 3.01 x 1022 molecules (c) 3.23 x 1025 molecules How many formula units are in: (a) 20g of sodium bromide? (b) 0.44g of potassium oxide? (c) 120g of ammonium nitrate? Answers (a) 1.17 x 1023 f.u. (c) 9.03 x 1023 f.u. (b) 2.82 x 1021 f.u. Mass to Number of Atoms or Ions How many atoms are in 2.4g of water? Number of moles of water = mass mass 1 mole = 2.4 18 = 0.13 moles 1 molecule of water = 1 mole of molecules = 3 atoms 3 moles of atoms Number of atoms = moles x atoms in 1 mole = 0.13 x 3 x 6.02 x 1023 = 2.41 x 1023 atoms How many atoms are in: (a) 0.23g of methane? (b) 6.9g of carbon dioxide? (c) 100g of ammonia? Answers (a) 4.33 x 1022 atoms (b) 2.83 x 1023 atoms (c) 1.42 x 1025 atoms How many ions are in: (a) 3g of lithium fluoride? (b) 0.072g of aluminium chloride? (c) 4.8kg of lead(IV) oxide? Answers (a) 1.39 x 1023 ions (b) 1.30 x 1021 ions (c) 3.63 x 1025 ions Mass to Number of Electrons or Protons Etc. How many electrons are in 1g of lithium? Number of moles of lithium 1 atom of lithium 1 mole of lithium Number of electrons = mass mass 1 mole = 1 7 = 0.14 moles = = 3 electrons 3 moles of electrons = moles x electrons in 1 mole = 0.14 x 3 x 6.02 x 1023 = How many electrons are in: 2.58 x 1023 electrons (a) 0.02g of carbon? (b) 3g of water? (c) 0.65g of oxide ions? Answers (a) 6.02 x 1021 electrons (b) 1.00 x 1024 electrons (c) 2.45 x 1023 electrons How many protons are in: (a) 4g of argon? (b) 0.016g of ethanol? (c) 19.2g of potassium ions? Answers (a) 1.08 x 1024 protons (b) 5.44 x 1021 protons (c) 5.63 x 1024 protons Number to Mass What mass of carbon contains 1.25 x 1022 atoms? Number of moles of atoms Mass of carbon = = = = atoms in question atoms in 1 mole = 1.25 x 1022 6.02 x 1023 = 0.021 moles moles x mass 1 mole 0.021 x 12 0.25g What mass of: (a) carbon monoxide contains 3.46 x 1025 molecules? (b) ammonia contains 2.34 x 1024 atoms? (c) methane caontains 6.75 x 1022 hydrogen atoms? (d) potassium carbonate contains 3.35 x 1022 f.u.s? (e) calcium iodide contains 6.71 x 1021 ions? (f) magnesium nitrate contains 7.01 x 1024 nitrate ions? (g) boron contains 3.67 x 1021 electrons? (h) hydrogen sulphide contains 5.42 x 1025 electrons? Answers (a) 1.61kg carbon monoxide (b) 16.52g ammonia (c) 0.45g methane (d) 7.68g potassium carbonate (e) 1.09g calcium iodide (f) 862g magnesium nitrate (g) 0.013g boron (h) 170g hydrogen sulphide Molar Volume The molar volume is the volume of 1 mole of a gas The molar volume can be calculated from the density of the gas using the formula: density = mass volume For example: What is the molar volume of ammonia [density = 0.771g l-1] From the above equation: volume = Mass of 1 mole = 17g Volume of 1 mole = 17 0.771 = 22.05 litres = 22.05 l mol-1 The molar volume mass density Calculate the molar volume of: (a) Argon [density = 1.784g l-1] (b) Carbon monoxide [density = 1.250g l-1] (c) Hydrogen sulphide [density = 1.539g l-1] Answers (a) 22.42 l mol-1 (b) 22.40 l mol-1 (c) 22.09 l mol-1 At the same temperature and pressure, the molar volume of any gas is the same. Gas Volume to Number How many molecules are in 0.4 litres of carbon dioxide gas if the molar volume is 24.3 l mol-1? Number of moles = volume molar volume = 0.4 24.3 = 0.016 moles Number of molecules = moles x molecules in 1 mole = 9.91 x 1021 molecules (a) How many molecules are in 6.2 litres of hydrogen fluoride if the molar volume is 27.6 l mol-1? (b) How many atoms are in 1.5 litres of methane if the molar volume is 20.5 l mol-1? (c) How many oxygen atoms are in 250 cm3 of sulphur trioxide if the molar volume is 23 l mol-1? Answers: (a) 1.35 x 1023 molecules (b) 2.20 x 1023 atoms (c) 1.96 x 1022 oxygen atoms Number to Volume What volume of argon contains 5.43 x 1020 atoms if the molar volume is 23.7 l mol-1? Number of moles Volume of argon = atoms atoms in 1 mole = 5.43 x 1020 6.02 x 1023 = 9.01 x 10-4 moles = moles x molar volume = 9.01 x 10-4 x 23.7 = 0.021 litres = 21.38 cm3 (a) What volume of chlorine contains 3.25 x 1022 molecules if the molar volume is 24.5 l mol-1? (b) What volume of sulphur dioxide contains 5.52 x 1023 atoms of sulphur if the molar volume is 26.2 l mol-1? (c) What volume of ammonia contains 8.42 x 1021 atoms of hydrogen if the molar volume is 22.8 l mol-1? Answers (a) 1.32 litres of chlorine (b) 24.02 litres of SO2 (c) 106 cm3 of ammonia Volume and Particles How many ions are in 50cm3 of 1M (1 mol l-1) sodium chloride solution? Number of moles = concentration x volume = 1 x 0.05 = 0.05 moles 1 NaCl formula unit = 1 mole of NaCl f.u. = 2 ions 2 moles of ions Number of ions = moles x ions in 1 mole = 0.05 x 2 x 6.02 x 1023 = 6.02 x 1022 ions (a) How many ions are in 2 litres of 0.25M magnesium bromide solution? (b) How many potassium ions are in 3.2 litres of 0.01M potassium sulphate solution? (c) How many nitrate ions are in 150cm3 of 2M aluminium nitrate solution? Answers (a) 9.03 x 1023 (b) 3.85 x 1022 (c) 5.42 x 1023 Particles to Volume/Concentration What volume of lithium bromide solution contains 2.34 x 1022 ions if the concentration is 0.02M? Number of moles = particles particles in 1 mole = 2.34 x 1022 2 x 6.02 x 1023 = 0.019 moles Volume of solution = moles concentration = 0.019 0.02 = 0.972 litres (a) What volume of 0.125M sodium hydroxide solution contains 8.72 x 1021 ions? (b) What volume of 2.4M calcium chloride solution contains 5.54 x 1025 chloride ions? (c) What concentration of potassium carbonate solution contains 4.77 x 1022 ions in 25cm3? (d) What concentration of barium nitrate solution contains 3.97 x 1023 nitrate ions in 1.6 litres? Answers (a) 57.9 cm3 (d) 0.206M (b) 19.2 litres (c) 1.06M Equations and Volumes Remember At the same temperature and pressure, the molar volume of any gas is the same Using Balanced Equations A balanced equation shows the molar relationship between the reactants and products e.g. H2 1 mole + Cl2 1 mole 2HCl 2 moles So, 1 mole of hydrogen reacts with 1 mole of chlorine producing 2 moles of hydrogen chloride Since 1 mole of hydrogen has the same volume as 1 mole of chlorine: H2 1 volume + Cl2 1 volume 2HCl 2 volumes e.g. 3 100 cm 3 100 cm 3 200 cm Volumes of reactants and products can be worked out directly from the balanced equation provided they are in the gas state 3 What volume of hydrogen chloride is produced if 25 cm of hydrogen is reacted with excess chlorine? From the balanced equation: 1 vol H2 2 vol HCl 3 3 25 cm H2 2 x 25 cm HCl 3 50 cm HCl 3 (a) What volume of ammonia is produced when 40cm of nitrogen is reacted with excess hydrogen? N2(g) + 3H2(g) 2NH3(g) (b) What volume of hydrogen is required to produce 50cm of ammonia? 3 (c) What volume of carbon dioxide is produced when 3 15cm of methane is burned in excess oxygen? CH4(g) + 2O2(g) Answers (a) 80 cm 3 CO2(g) + 2H2O(l) 3 (b) 75 cm (c) 15 cm 3 Total Volumes The volume of solids and liquids is ignored when calculating volumes from equations What is the total volume of gas after the reaction if 100 cm 3 of carbon monoxide is reacted with 400 cm of hydrogen? 3 (i) write the balanced equation and volume relationship: CO(g) 1 vol + 3H2(g) 3 vol CH4(g) + 1 vol H2O(l) — (ii) convert to actual volumes: 100 cm 3 300 cm 3 3 — 100 cm 3 Volume of methane produced 100 cm Volume of unused hydrogen 400cm - 300cm 3 100 cm 3 200 cm Total volume of gases 3 3 (a) What is the total volume of gas produced when 50 cm 3 of carbon monoxide is burned in 50 cm of oxygen? 3 (b) What is the total volume of gas produced when 30 cm 3 of methane is burned in 100 cm of oxygen? 3 Answers 3 (a) 75 cm 3 (b) 70 cm Excesses If a reactant is in excess, it means that there is far more of it than is needed for the reaction Excess reactants can be determined from the reaction equation Which reactant is in excess (and by how much) when 10.5g 3 magnesium carbonate is reacted with 150 cm of 2M Nitric -1 acid? (2M = 2 mol l ) (i) write the balanced equation and molar relationship for the reaction: MgCO3(s) + 2HNO3(aq) 1 mol 2 mol Mg(NO3)2(aq) + CO2(g) + H2O(l) 1 mol 1 mol 1 mol (ii) write the relationship between the reactants: 1 mole MgCO3 reacts with 2 moles HNO3 (iii) work out the number of moles of reactants from the question: Number of moles of MgCO3 = mass mass 1 mole = 10.5 84.5 = 0.125 moles Number of moles of HNO3 = conc x volume = 2 x 0.150 (litres) = 0.30 moles So, If 1 mole MgCO3 0.125 moles MgCO3 2 moles HNO3 0.25 moles HNO3 Since, 0.25 moles HNO3 needed & 0.30 moles HNO3 available HNO3 is in excess by 0.05 moles (i.e. 0.30 - 0.25) (a) Which reactant is in excess (and by how much) if 6.5g 3 of zinc is reacted with 10 cm of 4M hydrochloric acid? (b) Which reactant is in excess (and by how much) if 4g of sulphur is reacted with 14g of Iron metal to produce iron sulphide? (c) Which reactant is in excess (and by how much) if 3 3 100cm of ethene is burned in 100cm of oxygen? Answers: (a) 0.02 moles excess Zinc 3 (b) 0.125 moles excess iron (c) 50cm excess ethene Percentage Yield Not all reactions work to give 100% products and the percentage yield shows the proportion of the reactants which are made into products What is the percentage yield when 21 litres of hydrogen -1 (molar volume = 25 l mol ) produces 1.19 g of ammonia? (i) write the balanced equation and molar relationship: N2(g) 1 mol + 3H2(g) 3 mol 2NH3(g) 2 mol (ii) work out the number of moles of hydrogen in question: Number of moles = = = 0.84 moles (iii) write the relationship between hydrogen and ammonia: 3 moles of hydrogen 0.84 moles of hydrogen 2 moles of NH3 2 x 0.84 moles of NH 3 3 0.56 moles of NH3 (iv) work out the mass of ammonia which should have been produced: Mass of ammonia (v) percentage yield = moles x mass 1 mole = 0.56 x 17 = 9.52g = = = 12.5% 3 (a) What is the percentage yield when 40 cm of ammonia 3 reacts to produce 30 cm of nitrogen monoxide? (b) What is the percentage yield when 3.84g of sulphur trioxide is formed from 1.8 litres of sulphur dioxide -1 (molar volume 30 l mol ) reacting with excess oxygen? Answer (a) 75% yield (b) 80% yield