Appendix A. Mixing Rules - University of Mississippi
advertisement

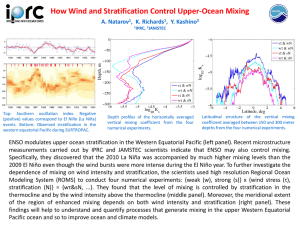
Mathcad Modules for Supercritical Fluid Extraction Based on Mixing Rules Appendix A – Mixing Rules Wei-Yin Chen Jiangping Liu Department of Chemical Engineering University of Mississippi 134 Anderson Hall University, MS 38677 Copyright© November 13, 2007 Corresponding author. Tel. (662) 915-5651; Fax. (662) 915-7023 E-mail address: cmchengs@olemiss.edu 1 Appendix A. Mixing Rules Several mixing rules have been employed for depicting the parameters a and b of a cubic EOS for mixtures. A complete discussion of these missing rules can be found in the book by Orbey and Sandler.[1] van der Waals Mixing Rules The traditional van der Waals one fluid mixing rules stipulate the parameters of a mixture through the following expressions: a xi x j aii a jj b xi x j 0.5 1 k (A-1) ij bii b jj (A-2) 2 In Eq. A-1, k ij is the binary interaction parameter, which is usually recovered by using the experimental data. Through the expression relating the parameters between a cubic EOS and a virial EOS, i.e., B a / RT , it can be shown that the van der Waals mixing rules conform to the statistical mechanics requirement of quadratic dependence of the second virial coefficient on composition. These mixing rules can be used to describe mixtures such as hydrocarbons, but not accurately for polar and associating compounds. With these mixing and combining rules and the PRSV EOS, the fugacity coefficient, 2G , in a homogeneous phase can be expressed as: 2 y j a2 j A b2 b2 G 2 exp Z 1 ln Z B j 1 b a 2 2B b 2 Z 1 2 B ln Z 1 2 B (A-3) Mixing Rules of Vidal and Huron (HV) Vidal and Huron[2] demonstrated that the traditional van der Waals mixing rules are consistent with the regular solution theory and has limited capability in fitting excess Gibbs energy data for polar species over a wide pressure range. They devised a procedure that successfully matched the excess Gibbs energy, G E , derived from an equation of state with that from an activity coefficient model at infinite pressure. Their procedure yielded a mixing rule with the parameters a and b in a cubic EOS: a GE a b xi i * bi C (A-4) b is written in a simple van der Waals form as Eq. A-2. C * is an EOS-dependent parameter.[3] For the PRSV EOS, this parameter is -0.62323. In matching G E , Vidal and Huron also assumed that V b , and V E 0 . The fugacity coefficient, 2G , for the HV model can be expressed by: ln 2 Z 1 2 B b2 1 a2 (A-5) exp Z 1 ln Z B ln b C * Z 1 2 B 2 2 b2 RT G 2 The novelty of the Vidal and Huron’s combination of an EOS and an activity (excess Gibbs model) has inspired a family of matching procedures over the last two decades. Of special interest is the use of predictive GE models in this EOS/GE models, such as the UNIFAC class, as it renders the EOS/GE algorithm totally predictive. Mixing Rules of Modified Huron-Vidal (MHV) Since the low-pressure group interaction parameters have been constantly updated for UNIFAC, the traditional UNIFAC may not be adequate for a predictive EOS/G E 3 algorithm that was derived by matching GE at infinite pressure. As a remedy, Mollerup[4] proposed a procedure that matches excess free energies at zero pressure. Nevertheless, this procedure requires solving the molar volume of liquid species from an EOS, which is not always feasible, as the isotherms of a cubic EOS do not necessary cross the molar volume axis at high temperatures. To resolve this issue, Michelsen[5] developed a extrapolation procedure to approximate the molar volume at zero pressure. The mixing rules resulted from the matching at zero pressure include the parameter b in the same form as Eq. A-2, and the parameter a , a solution of the following expression: q xi q i i b GE xi ln RT i bi (A-6) where V 0 a q ln 1 C* b bRT (A-7) The superscript 0 for molar volume delineates its value at P 0 . Michelsen[6] approximated q by a polynomial function of a bRT , a dimensionless energy parameter. Specifically, in the so-called MHV1 model, Michelsen chose an a linear approximation for q , i.e., q q0 q1 (A-8) Later, in the so-called MHV2 model, a quadratic correlation was proposed for q for improved flexibility:[7] q q0 q1 q2 2 (A-9) 4 where the parameters q1 and q2 in the above expression are chosen to ensure continuity of q and its derivatives. The recommended values of q1 and q2 are -0.41754 and -0.00461, respectively for the PRSV EOS.[4] Substituting Eq. A-9 into Eq. A-6 yields: b GE q1 mix xi ii q2 ii2 xi ii2 xi ln mix RT bii i i (A-10) This process results in the following expression for fugacity coefficient: b2 1 N Z 1 2 B Z 1 ln Z B ln b 2 2 N 2 Z 1 2 B 2G exp (A-11) where b b q1 2 q2 ( 2 22 ) ln 2 ln 2 1 N b2 b N 2 q1 2q2 (A-12) Mixing Rules of Linear Combination of Huron-Vidal and Michelsen Models (LCVM) Boukouvalas et al.[8, 9] proposed a mixing rule by linearly combining the ’s of HV and MHV1 models, which results in the following mixing rule for a : 1 G E 1 b a a bRT * xi ln xi ln i q1 RT q1 i bi i bi RT C (A-13) where is an adjustable parameter that determines the relative importance of the combined rules. They have suggested the value = 0.36 for the original UNIFAC for low pressure systems, and values in the range from 0.65 to 0.75 for the modified UNIFAC for high pressure systems. The value of q1 is set equal to -0.52. Eq. A-2 is again for b . The fugacity coefficient resulted from this combination is 5 ln 2G b2 Z 1 ln Z B b 1 1 a2 1 b b2 Z 1 2 B ln ln 1 ln 2 * q1 q1 b2 b 2 2 b2 RT C Z 1 2 B (A-14) Mixing Rules of Wong and Sandler (WS) Although the traditional van der Waals mixing rules fail to model the excess Gibbs energy over a wide range of pressures, their quadratic dependence on compositions lead to a quadratic dependence of the second virial coefficient on composition for mixtures, which is stipulated by statistical mechanics. The mixing rules of Huron and Vidal are linear and do not satisfy the quadratic requirement. Wong and Sandler[10, 11] derived a general form of mixing rules first by combining the quadratic dependence of the second virial coefficient on composition and the relation between the second virial coefficient and the parameters in a cubic EOS. The second equation in their mixing rules was derived by taking the limit of the excess Helmholtz free energy for a cubic EOS mixture at infinite pressure. Helmholtz free energy is less pressure dependent, and can be approximated by excess Gibbs energy at low pressure where most experimental data are collected. The resultant mixing rules are pressure independent and satisfy the quadratic requirement: a RT xi x j b RT ij i j b a GE RT xi i * i bi C (A-15) GE a a b * xi i bi C (A-16) 6 where a j ai a 1 1 kij b bi bj RT ij 2 RT RT (A-17) ai a j a 1 b b b 1 kij i j RT ij 2 RT (A-18) or In our calculation, we use Eq. A-18. k ij is a second virial coefficient binary interaction parameter, it has to be obtained experimentally near the conditions of interests. The experimental dependence of k ij renders the WS model not a fully predictive scheme. The fugacity coefficient expression for the solid solute in supercritical extraction for the WS mixing rule is: Nb N 2 Z 1 ln Z B b 1 2 N N a N 2 Nb N 2 Z 1 2 B a ln a b 2 2bRT Z 1 2 B ln 2G (A-19) The partial derivatives in the above expression are Nb 1 1 N 2 a Q ND 1 N 2 1 D N N 2 1 D 2 N 2 (A-20) Nb ND 1 N 2 a RTD RTb N N 2 N 2 N 2 (A-21) a Q x2 x j b RT 2 j 2 j (A-22) and where 7 D a GE x2 2 * C RT b2 RT (A-23) 1 N 2Q a 2 x j b N N 2 RT 2 j j (A-24) ln 2 a ND 2 N 2 b2 RT C* (A-25) and Mixing Rules of Modified Huron-Vidal Model (HVOS) To approximate the molar volume of liquid at high pressures and high temperatures, Orbey and Sandler[12] assumed that there is an universal linear algebraic core volume as V ub , where u is a positive constant greater than unity. By matching the Helmholtz free energy at infinite pressure and assuming u 1 , they generated a new set of mixing rules, or the HVOS model in short, that involves parameter a in the following form: b a 1 GE a bRT xi i * xi ln bi RT C RT i bi i (A-26) and b is the same as Eq. A-2. The fugacity coefficient for the HVOS mixing rules for PRSV equation becomes ln 2G b2 Z 1 ln Z B b ln 2 1 b 1 b2 Z 1 2 B 1 a2 * ln * 1 ln C* C 2 2 b2 RT Z 1 2 B b2 C b 8 (A-27) Predictive Soave-Redlich-Kwon Model (PSRK) Gmehling et al [13-16] developed PSRK (Predictive Soave-Redlich-Kwong) model based on the Soave-Redlich-Kwong EOS and group contribution (modified UNIFAC). It could predict thermodynamic properties over a large temperature and pressure range, especially vapor-liquid equilibria at high pressures using UNIFAC parameters which were mainly fitted to vapor-liquid equilibrium data around atmospheric pressure and it continues to introduce new group and new group interaction parameters based on experimental data. The mixing rule is similar as MHV1 model: b ai 1 GE a bRT xi xi ln bi RT A0 RT i bi i (A-28) where A0 0.64663 and b is the same as Eq. A-2. References 1. Orbey, H., and S. I. Sandler, Modeling Vapor-Liquid Equilibria: Cubic Equations of State and Their Mixing Rules, Cambridge University Press, Cambridge, U.K. (1998) 2. Huron. M., and J. Vidal, “New Mixing Rules in Simple Equations of State for Representing Vapor-Liquid Equilibria of Strongly Non-ideal Mixtures,” Fluid Phase Equilibira, 3, 255-271 (1979) 3. Orbey. H., and S. I. Sandler, “Analysis of Excess Free Energy Based Equations of State Models,” AIChE Journal, 42(8), 2327-2334 (1996) 4. Mollerup, J. M., “A note on the derivation of Mixing Rules from Excess Gibbs Energy Models,” Fluid Phase Equilibria, 25, 323-326 (1986) 5. Michelsen, M. L., “A Modified Huron-Vidal Mixing Rule for Cubic Equations of State,” Fluid Phase Equilibria, 60, 213-219 (1990) 9 6. Span, R., and W. Wagner, “A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100K at Pressures up to 800 MPa,” Journal of Physical and Chemical Reference Data, 25(6), 1509-1596 (1996) 7. Dahl, S., A. Fredenslund, and P. Rasmussen, “The MHV2 Model: A UNIFAC-Based Equation of State Model for Prediction of Gas Solubility and Vapor-Liquid Equilibria at Low and High Pressures,” Ind. Eng. Chem. Res., 30, 1936-1945 (1991) 8. Boukouvala, C., N. Spiliotis, P. Coutsikos, and N. Tzouvaras, “Prediction of VaporLiquid Equilibrium with the LCVM Model: A Linear Combination of the Vidal and Michelsen Mixing Rules Coupled with the Original UNIFAC and the t-mPR Equation of State,” Fluid Phase Equilibria, 92, 75-106 (1994) 9. Yakoumis, I. V., and V. Konstantinos, “Application of the LCVM Model to Systems Containing Organic Compounds and Supercritical Carbon Dioxide,” J. Supercrit. Fluids., 9, 88-98 (1996) 10. Wong, D. S. H., and S. I. Sandler, “A Theoretically Correct Mixing Rule for Cubic Equations of State,” AIChE J., 38(5), 671-680 (1992) 11. Wong, D. S. H., H. Orbey, and S. I. Sandler, “Equation of State Mixing Rule for Nonideal Mixtures Using Available Activity Coefficient Model Parameters and That Allows Extrapolation Over Large Ranges of Temperature and Pressure,” Ind. Eng. Chem. Res., 31(8), 2033-2039 (1992) 12. Orbey, H., and S. I. Sandler, “On the Combination of Equation of State and Excess Free Energy Models,” Fluid Phase Equilibria, 111, 53-70 (1995c) 13. Holderbaum, T., and J. Gmehling, “PSRK: A Group Contribution Equation of State Based on UNFAC,” Fluid Phase Equilibria, 70, 251-265 (1991) 14. Ahlers, J., T. Yamaguchi, and J. Gmehling, “Development of a Universal Group Contribution Equation of State. 5. Prediction of the Solubility of High-Boiling Compounds in Supercritical Gases with the Group Contribution Equation of State Volume-Translated Peng-Robinson,” Ind. Eng. Chem. Res., 43(20), 6569-6576 (2004) 15. Gmehling. J., J. D. Li, and S. Martin, “A Modified UNIFAC Model. 2. Present Parameter Matrix and Results for Different Thermodynamic Properties,” Industrial and engineering chemistry research, 32,178-193 (1993) 16. Gmehling, J., “Company Consortium for the Revision, Extension and Further Development of the Group Contribution Methods UNIFAC, Mod. UNIFAC (Do) and the Predictive Equation of State PSRK,” <http://134.106.215.86/UNIFAC/> (2006) 10