Transport and Reaction Equation
advertisement
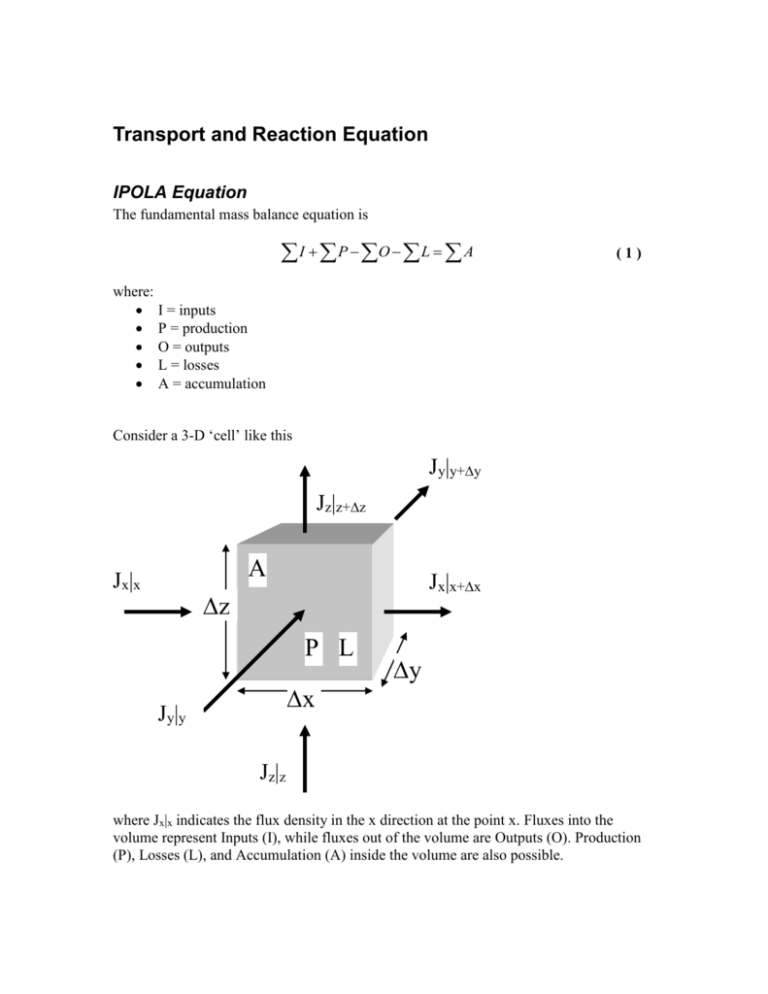
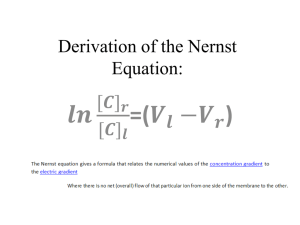
Transport and Reaction Equation IPOLA Equation The fundamental mass balance equation is I P O L A (1) where: I = inputs P = production O = outputs L = losses A = accumulation Consider a 3-D ‘cell’ like this Jy|y+y Jz|z+z A Jx|x Jx|x+x z P L x Jy|y y Jz|z where Jx|x indicates the flux density in the x direction at the point x. Fluxes into the volume represent Inputs (I), while fluxes out of the volume are Outputs (O). Production (P), Losses (L), and Accumulation (A) inside the volume are also possible. The cell can represent a small piece of many different things; it might represent a volume of water in a pond, part of the atmosphere, a portion of an aquifer, or a block of steel. Similarly, the flux density J might be a liquid flow, a heat (energy) flux, or a chemical flux. Since our primary interest is in the movement of chemicals in the environment, we’ll consider only the flux of chemical mass here. The approach has broad applicability though. Advective Flux Density The mass flux (ML-2T-1) can consist of a number of components. First, there may be a fluid (liquid or gas) flow with velocity v that carries along a mass of chemical. Let’s consider just the x direction. In time t, a velocity v (at x) will sweep in a volume V = v|x t y z. The volume swept out may be different because it depends on the velocity at x + x. The chemical mass going in or out in time t depends on the fluid volume and the concentration C in the fluid: Mass/Time = Concentration x Volume/Time. If we divide everything by the area of the inflow (or outflow) faces, we get the mass flux density, which ends up being very simple: Jx|x = C v where C is concentration and v is the velocity at point x. Diffusive/Dispersive Flux A second possible contribution to Jx|x is diffusion or dispersion. We know from Fick’s Law that the diffusive/dispersive flux density (in the x direction at point x) is J x x D C x (2) x Similar expressions can be written for the diffusive/dispersive fluxes in the y and z directions and at the other edges of the control volume. Total Input/Output Fluxes The advective and diffusive/dispersive fluxes can simply be added together to get the totals. We’ll have one flux for each face of the control volume (6 altogether). Three (at points x, y, and z) will be inputs and 3 (at x + x, y + y, and z + z) will be outputs: Inputs J x x Cvx x D Jy y C x Cv y D y J z z Cvz z D (3) x C y C x (4) y (5) z Outputs Jx Jy Jz x x Cvx y y Cv y z z Cvz x x y y z z D C x x x C y y y C z z z D D (6) (7) (8) Production and Losses Production of a particular chemical species can be the result of a chemical reaction. For example, nitrification of organic forms of nitrogen results in the production of nitrate (NO3-). Losses can be due to radioactive decay for example. We will consider two types of reactions that lead to production or losses. Zero order reactions proceed at a constant rate, with chemical mass being produced or lost. The reaction rate dC/dt = k0 (a constant) is given as M L-3 T-1 or mols L-3 T-1, i.e., per unit volume and time. dC/dt < 0 indicates loss, while dC/dt > 0 indicates production. If we multiply by the volume of our cell (xyz), we can get the total mass rate of loss or production in the cell. First order reactions are characterized by rates that depend on the concentration present. For these reactions C k1C t (9) Accumulation The rate of change of chemical mass in the control volume is CV/t where C is the change in concentration over the time t. If we replace the volume V by xyz and use small C and t, we can express the accumulation A in terms of the change in concentration over time: A C xyz t ( 10 ) Putting it all together Start with IPOLA: I P O L A ( 11 ) To get the total amounts coming in through a face we have to multiply the flux densities by the area of the face (yz for the x direction, xz for the y direction, and xy for the z direction). The sum of the inputs is C C Cv D y z Cv y y D xx x x y C xz Cvz z D xy x z y ( 12 ) and the sum of the outputs is Cv x Cv z x x z z D C x C D z y z Cv y x x xy z z IPOLA uses I – O, so we end up with y y D C y xz y y ( 13 ) C C C xz Cv z z D Cv x x D yz Cv y y D xy x x y y x z C C Cv x x x D y z Cv D x z y y y x x x y y y C Cv z z z D xy z z z ( 14 ) We can simplify this by collecting terms that have identical factors: Cv x x Cv x x x Cv y Cv y y Cv z z Cv z D y y z z C C D x x x D D yz x x C C D y y y C C D x z z xz y y ( 15 ) xy z z That is I – O. Let’s add the P (or L) and A terms: C C D Cv x x Cv x x x D yz x x x x x C C Cv y Cv y D D xz y y y y y y y y C C Cv z z Cv z z z D D xy x z z z z C k 0 xyz k1Cxyz xyz t Now divide everything by xyz. ( 16 ) Cv x x Cv x x x D C C D x x x x x x Cv y y Cv y Cv z z Cv z k 0 k1C y y D C C D y y y y y y z z D C C D x z z ( 17 ) z z z C t Look at the first part of the first term: Cv x x Cv x x x ( 18 ) x If we write this as Cv x x x Cv x x x ( 19 ) we see that it is the negative of the slope (gradient) of Cv: Cv|x Cv|x+x x x +x Hence, for infinitesimal x, we can write it as the derivative Cv t ( 20 ) Similarly, the second part of the first term is C C D D x x x x or x x ( 21 ) C D x D x x C x x ( 22 ) x ∂C/∂x|x x ∂C/∂x|x+x x +x We are taking the gradients of the gradients, and if we shrink x and y to differential size we have C D x 2C D 2 x x ( 23 ) Of course, there are similar expressions for the y and z directions. Using them in ( 17 ) yields Cvx Cv y Cvz 2C 2C 2C C D 2 2 2 k 0 k1C y z t y z x x ( 24 ) This is the advection diffusion reaction equation. It can take various forms depending on the details of the system, but overall it will have a form similar to ( 24 ). In one dimension with constant flow velocity ( 24 ) simplifies to C 2C C v D 2 k 0 k1C x t x ( 25 ) This can often be used if the coordinate system is carefully chosen. There are some analytical solutions of this equation but for many situations numerical solutions are required.