TAP512-0: Nuclear equations
advertisement

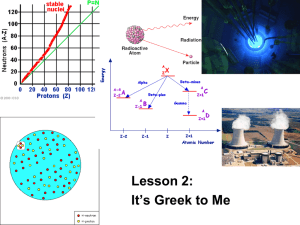
Episode 512: Nuclear equations Now that your students are familiar with different types of radiation, you can look at the processes by which they are emitted. Summary Discussion: Nuclide notation and N-Z plot. (10 minutes) Student Questions: Practice with notation. (10 minutes) Worked Examples: Equations for alpha, beta and gamma decay. (20 minutes) Student Questions: Practice with nuclear equations. (30 minutes) Discussion: Nuclide notation Revise nuclide notation: A Z X Discuss how A = mass or nucleon number, Z = charge or atomic number and N = neutron number are related (A = Z + N). Discuss isotopes (common examples: H, D and T, U-235 and U-238, C-14 and C-12). Set the task of finding out the name for nuclides having the same A but different Z (isobars), and the same N but different Z (isotones). Show an N-Z plot (Segrè plot). Neutron number n p + - + A + B p n + + + Proton number 1 Student questions: Practice with notation Set some simple questions involving nuclide notation. TAP 512-1: Nuclide notation Grid showing change in A and Z with different emissions A, Z-1 Nucleon number A, Z A, Z+1 (A) - + A-4, Z-2 Proton number (Z) Neutron number (N) + N+1, Z-1 N, Z - N-2, Z-2 N-1, Z+1 2 Proton number (Z) Worked examples: Equations for alpha, beta and gamma decay Nuclear decay processes can be represented by nuclear equations. The word equation implies that the two sides of the equation must ‘balance’ in some way. TAP 512-2: Decay processes You could give examples of equations for the sources used in school and college labs. sources are americium-241, 241 95 Am 4 Am 237 93 Np 2 He. - sources are strontium-90, 90 38 241 95 Sr 90 39 Np 0 1 90 38 Sr e. The underlying process is: n –> p + e- + Here, is an antineutrino. Your specification may require you to explain why this is needed to balance the equation. –> p + e- into the AZ notation: 1 0 n 11 H 0 1 e. sources are cobalt-60 60 60 27 decay of the 27 Co . The Co . The radiation comes from the radioactive daughter 60 28 Ni of the 60 28 Ni is formed in an ‘excited state’ and so almost immediately loses the energy by emitting a or decay, and all such rays have a well-defined energy. (So a cobalt-60 source which is a pure gamma emitter must be designed so that betas are not emitted. How? – (by encasing in metal which is thick enough to absorb the betas but which still allows gammas to escape.) Student questions: Practice with nuclear equations TAP 512-3: Practice with nuclear equations The more unusual decay processes (positron emission, neutron emission, electron capture) could be included, and students challenged to write them as nuclear equations. 3 TAP 512-1: Nuclide notation A periodic table may be needed A 1 Write down the nuclear notation ( Z X ) for: (a) an alpha particle (b) a proton (c) a hydrogen nucleus (d) a neutron (e) a beta particle (f) a positron. A 2 Write down the nuclear notation ( Z X ) for (a) carbon 13 (b) nitrogen 14 (c) neon 22 (d) tin 118 (e) iron 54 4 Answers 1 4 2 He (a) an alpha particle (b) a proton (c) a hydrogen nucleus (d) a neutron (e) a beta particle (f) a positron. 1 1 H or 11 p 1 0 1 1 H n 0 1 0 1 e e 2 13 6 (a) carbon-13 (b) nitrogen-14 (c) neon-22 (d) tin-118 118 50 (e) iron-54 54 26 22 10 C 14 7 N Ne Sn Fe 5 TAP 512-2: Decay processes Radioactive decay processes decay Z N Z–2 N–2 2 fewer protons 2 fewer neutrons proton number Z – decay – Z N Z+ 1 N–1 1 more proton 1 less neutron proton number Z + decay + Z–1 N+1 Z N 1 less proton 1 more neutron proton number Z decay Z N same protons and neutrons proton number Z 6 External reference This activity is taken from Advancing Physics chapter 18, 90O 7 TAP 512-3: Practice with nuclear equations 1 The isotope element? 235 U decays into another element, emitting an alpha particle. What is the This element decays, and the next, and so on until a stable element is reached. The complete list of particles emitted in this chain is: 235 92 U [, , , , , , , , , , ] X. What is the stable element X? (You could write down each element in the series, but there is a quicker way.) 2 The following fission reaction can take place in a nuclear reactor: 235 1 137 95 1 92 U 0 n 55 Cs [ ] Rb 0 n. Complete the equation, showing how many neutrons are produced in the reaction. What is the significance of the number of neutrons produced? Why are the products of the reaction, caesium-137 and rubidium-95, likely to be radioactive? What type of decay are these isotopes likely to show? 3 Boron absorbs neutrons with results as follows: 10 5 B 01 n 73 Li 24 . Why is boron suitable for use in a control rod? 4 27 When the isotope 13 Al is irradiated with alpha particles, the products from each aluminium nucleus are a neutron, and a nuclide that emits positrons to give the stable 30 isotope 14 Si . Write nuclear equations for these two processes. 8 5 Complete the following nuclear equations. In each case describe the decay process: 131 53 I Xe 67 0 31Ga 1e 11 6C Zn B 01e 99m 43Tc 6 0 1e Tc . The Manhattan Project, the development of the atomic bomb, led to the discovery of the transuranic elements (elements beyond uranium in the periodic table). Plutonium, element 94, is formed by the bombardment of uranium-238 with neutrons. The nuclear equations are: 238 1 239 92 U 0 n 92 U 239 239 0 92 U 93 Np 1 e 239 239 0 93 Np 94 Pu 1 e Complete the following nuclear equation for the formation of americium: 239 94 Pu 201 n Am 0 1e. Curium is produced if plutonium-239 is bombarded with alpha particles. If the curium 242 isotope is 96 Cm , complete the equation 239 94 Pu 4 2 If curium is made the target for alpha particle bombardment californium is produced. Complete the nuclear equation to find the atomic number of californium: 242 96 Cm 4 245 Cf 2 01n. By firing heavier particles such as carbon or boron ions at the target materials heavier elements can be synthesised. Complete the nuclear equation (Lw is lawrencium) 252 Cf 9 5B Lw 4 01 n. One of the transuranic elements is commonly found in the home. Which is this and where is it used? 9 Practical advice These questions are intended for homework or practice in class. Answers and worked solutions The complete decay chain involves the loss of seven alpha particles ( 2 ) and four beta 4 1. 0 e particles ( 1 ). This represents a loss of 7 4, i.e. 28, in mass number and (7 2 – 4), i.e. 10, in atomic number. X is therefore an isotope with mass number (235 – 28), i.e. 208 207, and atomic number (92 – 10), i.e. 82. This is lead, 82 Pb . 2. 235 92 U 1 137 0 n 55 Cs 95 37 Rb 3 01 n. The reaction produces more neutrons than it absorbs, this will cause a ‘chain reaction’. To see why the products of the reaction are likely to be radioactive you need to consult the plot of neutron number against atomic number for the known stable nuclei. For elements with atomic numbers up to about 30 the number of neutrons in the nucleus is the same as the number of protons if the nucleus is stable. For higher atomic numbers the ratio of neutrons to protons gradually increases to 1.5. Look at the position of both 137 95 55 Cs and 37 Rb on the plot and you will see that they both have a considerable excess of neutrons. They are therefore likely to be radioactive. To become more stable the nuclides need to decrease the neutron to proton ratio. The emission of a beta particle does this, increasing the number of protons by one and decreasing the number of neutrons by one. These isotopes are therefore likely to decay by emitting beta particles. 3. When boron captures a neutron it is transformed into a stable isotope. If the control rods are pushed into the reactor more neutrons are absorbed, causing the chain reaction to slow down. If they are pulled out the chain reaction will proceed more vigorously. 4. 27 13 Al 30 15 P 4 2 30 14 Si 30 15 P 1 0n 0 1e 5. 131 131 53 I 54 Xe 11 11 6 C 5 B 0 1e 0 1e beta decay positron emission 67 0 67 electron capture 31 Ga 1e 30 Zn 99m 99 nuclear rearrangem ent 43 Tc 43 Tc 6. 239 1 241 94 Pu 2 0 n 95 Am 239 4 242 94 Pu 2 96 Cm 242 4 245 96 Cm 2 98 Cf 252 98 Cf 9 257 5 B103 Lw 0 1e 1 0n 1 0n 4 01 n. Americium is commonly found in homes where it is used in smoke detectors. External reference This activity is taken from Advancing Physics chapter 18, 210S 10