mrnotes4 - University of Warwick
advertisement
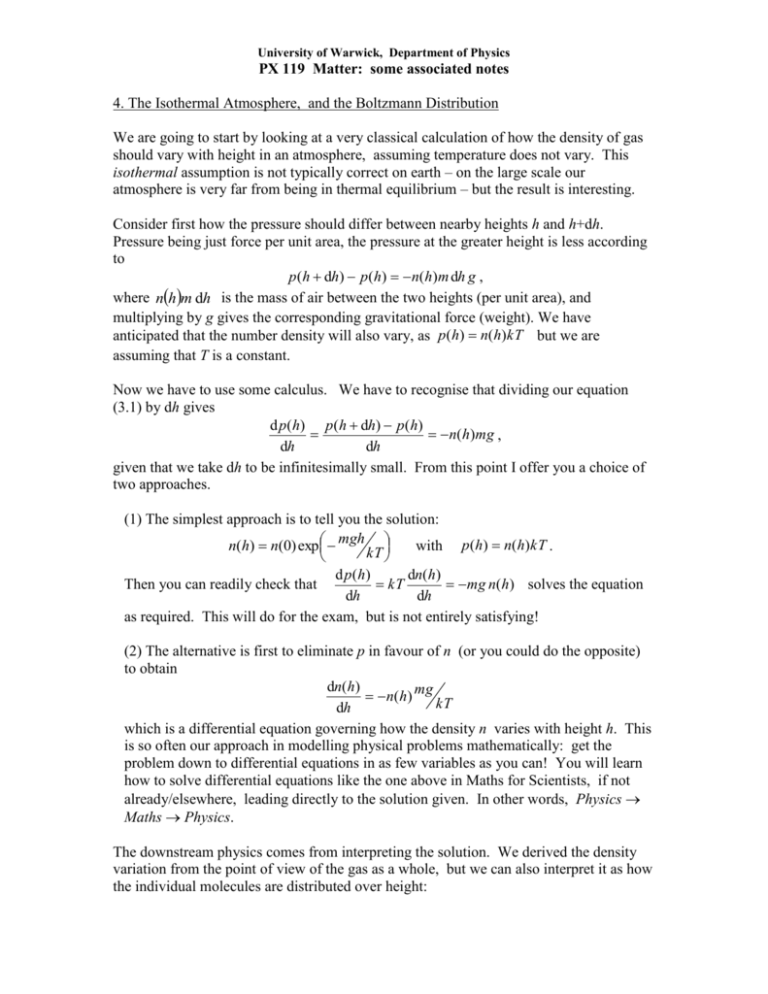
University of Warwick, Department of Physics PX 119 Matter: some associated notes 4. The Isothermal Atmosphere, and the Boltzmann Distribution We are going to start by looking at a very classical calculation of how the density of gas should vary with height in an atmosphere, assuming temperature does not vary. This isothermal assumption is not typically correct on earth – on the large scale our atmosphere is very far from being in thermal equilibrium – but the result is interesting. Consider first how the pressure should differ between nearby heights h and h+dh. Pressure being just force per unit area, the pressure at the greater height is less according to p(h dh) p(h) n(h)m dh g , where nhm dh is the mass of air between the two heights (per unit area), and multiplying by g gives the corresponding gravitational force (weight). We have anticipated that the number density will also vary, as p(h) n(h)kT but we are assuming that T is a constant. Now we have to use some calculus. We have to recognise that dividing our equation (3.1) by dh gives d p(h) p(h dh) p(h) n(h)mg , dh dh given that we take dh to be infinitesimally small. From this point I offer you a choice of two approaches. (1) The simplest approach is to tell you the solution: n(h) n(0) exp mgh with p(h) n(h)kT . kT d p ( h) dn(h) kT mg n(h) solves the equation Then you can readily check that dh dh as required. This will do for the exam, but is not entirely satisfying! (2) The alternative is first to eliminate p in favour of n (or you could do the opposite) to obtain dn(h) n(h) mg kT dh which is a differential equation governing how the density n varies with height h. This is so often our approach in modelling physical problems mathematically: get the problem down to differential equations in as few variables as you can! You will learn how to solve differential equations like the one above in Maths for Scientists, if not already/elsewhere, leading directly to the solution given. In other words, Physics Maths Physics. The downstream physics comes from interpreting the solution. We derived the density variation from the point of view of the gas as a whole, but we can also interpret it as how the individual molecules are distributed over height: University of Warwick, Department of Physics PX 119 Matter: some associated notes f (h) exp E (h) kT where f (h) is the fraction of molecules at height h, and E (h) mgh is the energy (gravitational potential energy) for a molecule to be at height h. What we have derived here is an example of the Boltzmann Distribution first shown in lecture 1, this time applied to just one molecule at a time. Applications of the Boltzmann Distribution Let us temporarily digress away from gases, to look at the simplest application of the Boltzmann distribution. Suppose we have a system which has just two possible states, which we can label ‘0’ and ‘1’. The spin of an electron aligned with or against a magnetic field is a standard example, but all we need here is that the states have energies E0 and E1 . According to the Boltzmann Distribution, the probability to find the system in state 0 is just p0AeE0kT and the probability to be in state 1 is p1AeE1kT. The constant of proportionality A has to be found by correctly normalizing the sum of probabilities so that p0 p1 1 leading to A 1 E0 kT . Now we know everything about the probabilities, e e E1 kT and for example we can calculate the average energy of the system as a function of temperature: E e E0 kT E1 e E1 kT E p0 E0 p1 E1 0 E0 kT . e e E1 kT For the distribution of molecules in the atmosphere, we have to integrate over heights rather than summing over a finite number of states. The BD says the probability to find a particular molecule at height h is given by f (h) A e mgh kT where the prefactor takes the value A mg kT in order that f ( h) d h 1 . Then, for 0 example, we can calculate the mean height of a molecule as kT h h f ( h) d h mg 0 and if you put in numbers for N2 in our atmosphere you should find a sensible value (isothermal is not such a bad approximation here). The Boltzmann distribution applies to velocities as well as positions (it is a fundamental principal of mechanics that velocities – strictly, momenta – can be treated in the same manner as positions). Therefore the probability for a molecule to have x-component of velocity with value vx is given from the kinetic energy by University of Warwick, Department of Physics PX 119 Matter: some associated notes 12 mv x 2 . f (v x ) A exp kT As before, the prefactor is fixed so that f (v x ) d v x 1 , giving A m 2kT in this case. (trust me on the integrals here – we will do go through this type later). Now it is particularly interesting to calculate the mean kinetic energy, because we already saw how this must come out for kinetic theory to match the ideal gas equation. Doing the required integral (again, trust me for the moment) over the distribution gives 1 2 mv x 2 1 2 mv x f (v x ) d v x 12 kT 2 which of course is the answer we wanted before. Equipartition Theorem and Heat Capacities of Ideal Gases If you look again at our result for the mean kinetic energy associated with v x you will see that although the mass m appears as a coefficient in the original expression for kinetic energy, it cancels out of the average. This is an example of the quite general Equipartition Theorem: suppose we have some coordinate (position or velocity) s and the associated energy takes the form E ( s) as 2 ; then the average of this energy in thermal equilibrium is given by E (s) 12 kT . Now I owe you a proof this time! The Boltzmann Distribution gives us f (s) A e as 2 / kT and this is correctly normalised using A 1 e as 2 / kT d s . Hence E E (s) as 2 e as 2 / kT ds e (**) as 2 / kT ds Maths! This ratio of integrals can be greatly simplified by rescaling the variables, guided by the form of the exponent. Let u s a in terms of which kT kT u 2 e u d u 2 E E ( s) e u 2 du ratio of 1 kT . kT standard integrals 2 The standard integrals are just numbers. For interest but not for examination, their ratio is easily shown to be 12 using integration by parts as follows. University of Warwick, Department of Physics PX 119 Matter: some associated notes d u u Noting that d e u 2ue u we have 2 u 2 e u 2 2 1 2 e u 2 1 2 e u 2 2 1 d u 0 e u d u 2 The full Equipartition Theorem is a slightly more general result, but follows obviously. Suppose the energy is written as a sum of several terms squaring different variables, for example E ( x, y, z ) ax 2 by 2 cz 2 . Then the full distribution over x, y and z (jointly) is given by ax 2 by 2 cz 2 ax 2 by 2 cz 2 exp exp exp f ( x, y, z ) exp kT kT kT kT which is just a product of independent distributions over x, y and z. Therefore each squared term in the energy can be averaged separately, giving us E 3 12 kT . The general statement is that if the energy is a sum of independent squares of coordinates and/or velocities, then the average of each term is 12 kT . A good example mixing coordinates and velocities is the Simple Harmonic Oscillator, for which the energy is E 12 cx 2 12 mv 2 , giving E 2 12 kT . Hence the heat capacity of a simple harmonic oscillator is given by CSHO k . Heat Capacities of the Ideal Gas Monatomic gas Recall the only energy here is Etranslational 12 m v x v x v x , associated with the kinetic energy of translation of the atom in three dimensions. We already saw that 5 5 3 CV k and hence C p CV k k , with the resulting ratio monatomic . 2 3 2 2 2 2 Diatomic Gas Now we have extra degrees of freedom in the molecule: two rotations plus one vibration. (The total has to be six for two atoms, each contributing three). k 2 2 The rotational kinetic energy is Erotations 12 I 1 2 leading to 2 contribution to 2 CV from rotational motion. 5 7 Thus without vibrations, we have CV k and hence C p CV k k , with the 2 2 7 resulting ratio diatomic without vibration . 5 University of Warwick, Department of Physics PX 119 Matter: some associated notes The diatomic molecule has one degree of freedom (bond stretch) which is a vibration. Because this has both potential and kinetic energy it contributes k to CV , leading to 9 diatomic with vibration . 7 Breakdown of Equipartition The Equipartition Theorem depends on classical mechanics, and so is only valid for motion contributing thermal energies large compared to quantum mechanical thresholds. In practice molecular rotations are quite classical at room temperature but vibrations in simple molecules are typically not. Thus we typically see the translational and rotational heat capacities as computed above, but not the full value for vibrational motion.