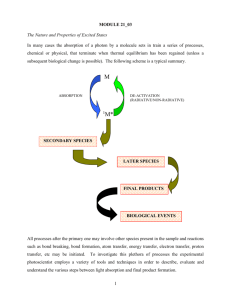
Luminescence spectroscopy
advertisement

Luminescence spectroscopy Emission techniques What happens to the molecule once it is electronically excited? The excess energy is transferred into vibration, rotation, and translation of surrounding molecules through collisions. � Molecule discards the excitation energy as a photon in a radiative decay process. � Fluorescence � Phosphorescence � The molecule dissociates and carries away the energy in the form of translation motion of the molecular fragments. Molecular Luminescence Spectroscopy Luminescence spectroscopy is a technique which studies the fluorescence, phosphorescence, and chemiluminescence of chemical systems. Fluorescence is light emission caused by irradiation with light (normally visible or ultraviolet light) and typically occurring within nanoseconds to milliseconds after irradiation. Phosphorescence is a light emission which can occur over much longer times (sometimes hours) after irradiation. It involves storage of energy in metastable states and its release through relatively slow (often thermally activated) processes. The phenomenon was discovered early on for phosphorus. Chemiluminescence is light emitted during (cold) chemical reactions. Fluorescence, phosphorescence, chemiluminescence all follow an electronic excitation. 1 Fig. 1. Time domain of fluorescence and phophorescence Electronic excited states are thermodynamically unstable. They persist for lifetimes that are normally about 10 nanoseconds for many medium sized organic molecules. A number of transition metal complexes have lifetimes of about 1 microsecond. Triplet states, resulting from a spin interconversion process in the excited state, have quite long lifetimes, sometimes to beyond 1 millisecond. Singlet and Triplet States Electrons in molecular orbitals are paired, according to Pauli exclusion principle. When an electron absorbs enough energy it will be excited to a higher energy state; but will keep the orientation of its spin. The molecular electronic state in which electrons are paired is called a singlet transition. On the other hand, the molecular electronic state in which the two electrons are unpaired is called a triplet state. The triplet state is achieved when an electron is transferred from a singlet energy level into a triplet energy level, by a process called intersystem crossing; accompanied by a flip in spin. 2 Fig. 2. Electronic transitions, paired electrons in ground and singlet state. In a singlet state, the spins of the two electrons are paired and thus exhibit no magnetic field and called diamagnetic. Diamagnetic molecules, containing paired electron, are neither attracted nor repelled by a magnetic field. On the other hand, molecules in the triplet state have unpaired electrons and are thus paramagnetic which means that they are either repelled or attracted to magnetic fields. The terms singlet and triplet stems from the definition of multiplicity where: Multiplicity = 2S + 1 Where, S is the total spin. The total spin for a singlet state is zero (-1/2, +1/2) since electrons are paired which gives a multiplicity of one (the term singlet state). Multiplicity = (2 * 0) + 1 =1 In a triplet state, the total spin is one (the two electrons are unpaired) and the multiplicity is three: Multiplicity = (2 * 1) + 1 = 3 It should also be indicated that the probability of a singlet to triplet transition is much lower than a singlet to singlet transition. Therefore, the intensity of the emission from a triplet state to a singlet state is much lower than emission intensities from a singlet to a singlet state. Energy Level Diagram for Photoluminescent Molecules The following diagram represents the main processes taking place in a photoluminescent molecule when it absorbs and emits energy. 3 Fig. 3. Schematic representation of different processes in luminescence transitions The different processes will be discussed below: 1. Absorption The absorption of UV-Vis radiation is necessary to excite molecules from the ground state to one of the excited states (S0 → S2). There are four different types of electronic transitions which can take place in molecules when they absorb UV-Vis radiation. A σ−σ* and a n−σ* are not useful for quantitative determination while the n−π* transition requires low energy but the molar absorptivity for this transition is low and transition energy will increase in presence of polar solvents. The most frequently used transition is the π−π* transition for the following reasons: a. The molar absorptivity for the π−π* transition is high allowing sensitive determinations. b. The energy required is moderate, far less than dissociation energy. c. In presence of the most convenient solvent (water), the energy required for a π−π* transition is usually smaller. Therefore, best molecules that may show absorption are those with π bonds or preferably aromatic nature as discussed earlier. Absorption to higher excited singlet states requires a very short time (in the range of 10-14 s). 2. Vibrational Relaxation (VR) Absorption of radiation will excite molecules to different vibrational levels of the excited state. This process is usually followed by successive vibrational relaxation as well as internal conversion (INC) to lower excited states. 4 INC: (An example of this process is the quinine sulfate fluorescence, which can be quenched by the use of various halide salts. The excited molecule can deexcite by increasing the thermal energy of the surrounding solvated ions.) In cases where transitions occur to the first excited state, vibrational relaxation to the main excited electronic level will take place and/or an intersystem crossing (ISC) to the triplet state can occur. 3. Fluorescence (FL) After vibrational relaxation to first excited electronic level takes place, a molecule can return to the ground state by emission of a photon, called fluorescence. The fluorescence lifetime is much greater than the absorption time and occurs in the range from 10-7 to 10-9s. As the lifetime in the excited state is increased, the probability of fluorescence will be decreased since radiationless deactivation processes may take place. However, not all excited molecules can show fluorescence by returning to ground state and most return to ground state by losing excitation energy as heat or through collisions with other molecules or solvent. Fig. 4. The mechanism of fluorescence. The initial excitation takes place between states of same multiplicity. The ground singlet state molecule, S0, goes into first excited singlet state, S1, Fluorescence may appear as an approximate mirror image of the absorption at a lower energy, and hence lower frequency (longer wavelength) than the absorption, the difference (in wavenumbers) between the two corresponding bands being known as the Stokes shift. Fluorescence emission may show vibrational structure which can provide information about the force constants of the molecule in its ground state (the 5 electronic structure provides information about the force constants in the excited state). Time of radiation = time of fluorescence. 4. Internal and External Conversion Internal conversion (INC) is a radiationless deactivation process whereby excited molecules return to the ground state without emission of a photon. This process seems to be the most efficient deactivation process in luminescence spectroscopy, since most molecules do not show fluorescence. However, molecules with close electronic energy levels, to the extent that their vibrational energy levels of ground and excited states are overlapped, are believed to cause efficient internal conversion. 5. Intersystem Crossing Electrons present at the first excited electronic level can follow one of three choices including emission of a photon to give fluorescence, radiationless deactivation to ground state, or intersystem crossing (ISC). The process of intersystem crossing involves transfer of the electron from an excited singlet to a triplet state. This process can actually take place since the vibrational levels in the singlet and triplet states overlap. However, crossing of the singlet state to the triplet state involves a flip in electron spin in order to satisfy the triplet state. Intersystem crossing is facilitated by presence of nonbonding electrons as well as heavy atoms. The presence of paramagnetic atoms or species also enhances intersystem crossing. An electron in the triplet state can also cross back to the singlet state and can result in a photon as fluorescence but at a much longer time than regular fluorescence. This process is termed delayed fluorescence and has the same characteristics as direct fluorescence except for the large increase in lifetime. 6. Phosphorescence Phosphorescence may be defined as the emission occurring due to the radiative transition between two states of different spin multiplicity, for example between T1 and S0. Electrons crossing the singlet state to the triplet state with a flipped spin can also follow one of three choices including returning to the singlet state (including a flip in spin), relax to ground state by internal or/and external conversion, or lose their energy as a photon (phosphorescence, Ph) and relax to ground state with a second flip in spin to satisfy the singlet ground state. As can be rationalized from the processes involved in collecting phosphorescence photons, this involves an intersystem crossing and two flips in spin. This, in fact, requires a much longer time than fluorescence (10-4 s to upto few s). Therefore, the probability of phosphorescence, and hence the intensity of the phosphorescence spectrum, is very low due to high possibility of radiationless deactivation. 6 Fig. 5. The mechanism of phosphorescence. Fig. 6. Phosphorescence occurs at a lower energy (lower wavenumber, longer wavelength) than fluorescence. Chemiluminescence: Chemiluminescence occurs when a chemical reaction produces an electronically excited species which emits a photon in order to reach the ground state. These sort of reactions can be encountered in biological systems; the effect is then known as bioluminescence. The number of chemical reactions which produce chemiluminescence is small. However, some of the compounds which do react to produce this phenomenon are environmentally significant. 7 A BC* D C* Ch Example: NO O3 NO2* O2 NO2* NO2 h(600 2800 nm) Used to detect NO from 1 ppb to 10 ppt. Intensity depends on rate of reaction of production of C* I cl k cl dc * dt 8 Reading Spin Orbit Coupling The magnetic interaction is responsible for spin orbit coupling. An electron is a charged particle so, its angular momentum from its orbit will result in a magnetic field. This orbital angular momentum (L) allows it to act like a tiny bar magnet we call spin angular momentum (S). Another magnet, the orbital angular momentumn (we refer to as quantum number L) can interact with the spin angular momentum. The interaction of these two magnets is called spin orbit coupling. a. A high total angular momentum corresponds to a parallel arrangement of magnetic moments (represented by bar magnets), and hence a high energy (J). We can think of it as a constructive interference. b. A low total angular momentum corresponds to a anti-parallel arrangement of magnetic moments and hence a low energy, interaction is destructive. Nuclei with a high number of protons will be at the centre of a strong magnetic current and the electron will experience a strong magnetic field. This strong field can cause the spin to flip. 9