SeversonRespiratory_System_LI
advertisement

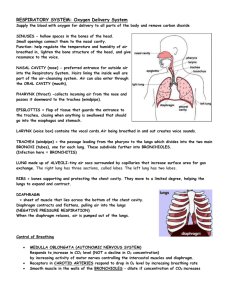
Med 6728 : Respiratory System : Development UM Medical School Duluth Fall Semester 2009 Department of Anatomy, Microbiology and Pathology Division of Anatomy and Cell Biology Dr. Arlen R. Severson Respiratory System Learning Issues – Normal and Abnormal Respiratory System Development 1. Describe the development of the lower respiratory organs beginning with the laryngotracheal groove and continuing through the respiratory diverticulum, tracheal bud, the tracheoesophageal folds and septum, and the development of the bronchi. (M & P, pp. 198-201) Laryngotracheal Groove: 28 days. Develops caudal to the 4th pair of pharyngeal pouches. It (which consists of endoderm) gives rise to the pulmonary epithelium & glands of the larynx, trachea, & bronchi. The CT, cartilage & SM develop from the splanchnic mesoderm surrounding the foregut. Respiratory Diverticulum: Evagination of laryngotracheal groove ventral to the caudal part of the foregut. Tracheal Bud: The diverticulum elongates & is invested with splanchnic mesenchyme. Its distal end enlarges to form this globular respiratory bud. Tracheoesophageal Folds: Longitudinal folds develop in the respiratory diverticulum (=laryngotracheal diverticulum). Tracheoesophageal Septum: Folds fuse to form a partition (septum). This septum divides the cranial portion of the foregut into a ventral part called the laryngotracheal tube (primordium of larynx, trachea, bronchi, & lungs) and a dorsal part (primordium of oropharynx & esophagus). Larynx: Epithelial lining develops from endoderm of cranial end of laryngotracheal tube. Cartilages from 4th & 6th pairs of pharyngeal pouches, which developed from mesenchyme that is derived from NC cells. Innervated by laryngeal branches of vagus nerves. Trachea: Epithelium & glands of the trachea & pulmonary epithelium develop from the endodermal lining of the laryngotracheal tube. Cartilage, CT, & muscles are derived from the splanchnic mesenchyme. Bronchi: Tracheal budprimary bronchial buds (grow laterally into the pericardioperitoneal canals, the primordial of the pleural cavitiessecondary & tertiary budstogether + splanchnic mesenchyme differentiate into bronchi. 2. What embryological tissue is responsible for the laryngeal cartilages and how are they associated muscles innervated? Cartilages from 4th & 6th pairs of pharyngeal pouches, which they themselves developed from mesenchyme that is derived from NC cells. Compare the developmental origin of structures distal to the larynx with that of the larynx. (M & P, pp. 198) The trachea develops distal to the larynx. Larynx Trachea (distal) Epithelial lining derived from endoderm of cranial foregut (laryngotracheal tube). Laryngeal cartilagesmesechymeNC cells Laryngeal muscles (skeletal)myoblasts of 4th & 6th pairs of pharyngeal arches (Laryngeal n) Hypobranchial eminenceepiglottis Epithelial lining is derived from endoderm of distal foregut (laryngotracheal tube). Cartilage splanchnic mesoderm. Muscles & CTsplanchnic mesoderm Glandsendoderm (ingrowth of surface epithelium) 3. Define a tracheoesophageal fistula (TEF) and the cause of its development. A fistula (abnormal passage) between the trachea & esophagus. A TEF results from incomplete division of the cranial part of the foregut into respiratory & esophageal parts during the 4th week. Incomplete fusion of the tracheoesophageal folds results in a defective tracheoesophageal septum & a TEF. Why may polyhydramnios occur in a baby with tracheoesophageal fistula? (M & P, pp. 198, 201, 202) Because fluid cannot pass to the stomach & intestines for absorption & subsequent transfer through the placenta to the mother’s blood for disposal resulting in this excess of amniotic fluid (polyhdramnios). 4. Describe the development of the bronchial buds and the significance of the pericardioperitoneal canals. (M & P, pp. 202-203) Tracheal budprimary bronchial buds (grow laterally into the pericardioperitoneal canals, the primordial of the pleural cavitiessecondary & tertiary budstogether + splanchnic mesenchyme differentiate into bronchi. Note: Splanchnic mesenchyme visceral pleura & somatic mesoderm parietal pleura. 5. Define the stages in development of the lungs, noting especially the development of alveoli and the formation of the blood-air barrier, and the cells involved. (M & P, pp. 203-207) What is the function of the cellular components of alveoli? Pseudoglandular Stage: 6 to 16 weeks At 16 weeks all major elements of lung formed, except those for gas exchange. A born fetus would not survive. Canalicular Stage: 16 to 26 weeks The lumina of the bronchi & terminal bronchioles become larger & lung tissue becomes highly vascular. Respiratory bronchioles form & each divides into three to six passages (primordial alveolar ducts). Some thin-walled terminal sacs (primordial alveoli) have developed at the ends of the respiratory bronchioles. Born fetus may survive with IC. Terminal Sac Stage: 26 weeks to birth Epithelium becomes very thin on developing terminal sacs. Capillaries slightly bulge into these sacs. This intimate contact between epithelial & endothelial cells establishes the blood-air barrier. Fetus would survive. Terminal sacs lined by squamous epithelial cells of endodermal origin – Type I pneumocytesacross which gas exchange occurs. Lymph vessels also develop. Type II penumocytes – scattered in squamous epithelium, secrete pulmonary surfactant (which forms a monomolecular film over alveolar sacs & counteracts surface T forces at the airalveolar interface; this helps expansion of saccules & prevents atelectasis). Surfactant production begins by 20 weeks and increases with pregnancy duration. By 26 to 28 weeks there is sufficient alveolar sacs and surfactant to permit survival. (Wt= 1000g). Use of antenatal corticosteroids, which induces surfactant production, & also with postnatal surfactant replacement therapy for treatment. Alveolar Stage: At 32 weeks sacs analgous to alveoli are present. The epithelial lining thins even more and type I pneumocytes become so thin that adjacent capillaries really bulge into them forming the alveolocapillary membrane (respiratory membrane), which allows gas exchange. The greatest increase in the size of the lungs after birth is due to the increase in respiratory bronchioles & primitive alveoli. 150 million alveoli 300 million alveoli at 3 years old. The primordial alveoli also enlarge as the lungs expand after birth. Breathing movements (FBMs) prior to birth help stimulate lung (by creating a P gradient btwn lungs & amniotic fluid) & respiratory musculature development. Some amniotic fluid is aspirated into the lungs during FBMs. At birth, the lungs approximately half-filled with fluid from the amniotic cavity, lungs, & tracheal glands. Aeration at birth is a rapid replacement of intra-alveolar fluid by air. The fluid in lungs is cleared at birth through the nose & mouth by P on the thorax during delivery; by pulmonary arteries, veins & caps; & by lymphatics. Note: 3 factors play into normal lung development: adequate thoracic space for lung growth, FBMs, & an adequate amniotic fluid volume. 6. Examine the following topics: oligohydramnios and lung development; lungs of a newborn; respiratory distress syndrome and its treatment; lung hypoplasia with congenital diaphragmatic hernia. (M & P, pp. 207) Oligohydramnios & Lung Development: When there is chronic or severe insufficient amount of amniotic fluid from leakage or decreased production, lung development is retarded & severe pulmonary hypoplasia develops from restriction of the fetal thorax. Lungs of a Newborn: Healthy lungs float because they contain some air. Stillborn lungs sink because they contain fluid. Respiratory Distress Syndrome & its Treatment: Prematures are most susceptible. After birth have rapid, labored breathing. The major cause is surfactant deficiency. Lungs are underinflated and contain a high protein content fluid that resembles a glassy or hyaline membrane, which is derived from circulation & from the injured pulmonary epithelium. It has been suggested that prolonged intrauterine asphyxia may produce irreversible changes in the type II alveolar cells, making them incapable of producing surfactant. Corticosterioids & thyroxine are potent stimulators of surfactnt production. Treatment: Maternal glucocorticoids accelerate fetal lung development & surfactant production. In preterm labor betamethasone (corticosteroid) can be sued to prevent RDS. Exogenous surfactant (surfactant replacement therapy) reduces the severity of RDS & neonatal mortality. Lung Hypoplasia with Congenital Diaphragmatic Hernia: The lung is unable to develop normally in infants with congenital diaphragmatic hernias because of compression by the abdominal viscera. Lung hypoplasia results reducing lung volume & hypertrophy of the smooth muscle in the pulmonary arteries also occurs. PHTN further leads too a decreased blood flow through the pulmonary vascular system as the blood continues to shunt thorugh the ductus arteriosus. Deaths are due to pulmonary insufficiency because lungs are too hypoplastic for air exchange & there is too much resistance for pulmonary blood flow to support extrauterine life. 7. Review the development of the diaphragm and how it’s development relates to the occurrence of congenital diaphragmatic hernia. (M & P, Chapter 8, pp. 150-155) Development of the Diaphragm: Septum transversum – primordium of the central tendon of the diaphragm – separates heart from liver, but does not completely separate the thoracic & abdominal cavities completely. Eventually fuses with the dorsal mesentery of the esophagus & the pleuroperitoneal membranes. Pleuroperitoneal membranes – fuse with the dorsal mesentery of the esophagus & the septum transversum completing the partition between the thoracic & abdominal cavities & forms the primordial diaphragm. Large part of diaphragm in early fetus, but a SMALL part in newborn’s diaphragm. Dorsal mesentery esophagus – Fuse with the septum transversum & pleuroperitoneal membranes. This mesentery constitutes the medial portion of the diaphragm. The crura of the diaphragm then develops from myoblasts that grow into the dorsal mesentery of the esophagus. Muscular ingrowth from lateral body walls – 9-12 weeks. Lungs & pleural cavities enlarge into the lateral body walls. Body wall tissues splits into two layers: External layer that becomes part of the definitive abdominal wall & Internal layer that contributes to peripheral parts of the diaphragm, external to the parts derived from the pleuorperitoneal membranes. Further extension of pleural cavities into lateral wall forms the costodiaphragmatic recesses. Congenital Diaphragmatic Hernia: Is usually, unilateral & results from defective formation &/or fusion of the pleuroperitoneal membrane with the other three parts of the diaphragm. Resulting in a large opening in the posterolateral region of the diaphragm. As a result, the peritoneal & pleural cavities are continuous with one another along the posterior body wall. Intestine, stomach, & spleen enter thorax and compress the development of the lungshypoplastic lungs which his often the cause of death. Study Guide (answers in bold) 1. Where does the laryngotracheal groove and diverticulum develop? From the ventral wall of the primitive pharynx caudal to the 4th pair of pharyngeal pouches. What germ layer contributes to the epithelial lining? Endoderm. What germ layer contributes to the supporting wall? Splanchnic mesenchyme. What is the purpose of the tracheoesophageal folds and septum? To divide the cranial part of the foregut into the laryngotracheal tube (primordium of the larynx, trachea, bronchi, & lungs) & dorsal portion (oropharynx & esophagus) 2. What branchial arch cartilages contribute to the laryngeal cartilages? 4th & 6th pairs of pharyngeal arches. What cells form the cartilaginous tissue? Neural Crest cells mesenchyme. What branchial arches give rise to the laryngeal muscles? 4th & 6th pairs of pharyngeal arches. What nerves innervate the laryngeal muscles? Superior laryngeal & recurrent laryngeal (vagus n). 3. What germ layers contribute to the laryngotracheal tube? Endoderm. 4. What germ layer gives rise to the tracheobronchial glands? Endoderm. What germ layer gives rise to the supporting walls? Splanchnic mesoderm. What tissue forms the visceral and parietal pleura? Visceralsplanchinic mesoderm & parietalsomatic mesoderm. 5. What developmental defect has occurred when a tracheoesophageal fistula is present? There is abnormal partitioning by the tracheoesophageal septum producing an abnormal communication between the trachea & esophagus. What is esophageal atresia? Where the superior part of the esophagus ends blindly. When would gastric secretions possibly cause pneumonitis? If the inferior part of the esophagus joined the trachea near its bifurcation & gastric & intestinal contents refluxed through the fistula into the trachea & lungs. When would lipid pneumonia occur? From aspiration of milk. When and why does polyhydramnios occur? Excess amniotic fluid develops because fluid cannot pass to the stomach & intestines for absorption & subsequent transfer through the placenta to the mother’s blood for disposal. 6. How do the lungs develop? Pseudoglandular (no gas exchange), canalicular (vascularized), terminal sac (blood-air barrier forms), & alveolar stages (caps, lymphatics, & gas-exchange). What is the usual cause of lung hypoplasia? Congenital diaphragmatic hernia. How may oligohydramnios cause lung hypoplasia? The fetal thorax is restricted from uterine wall compression caused by the amniotic fluid leakage or decreased production. Why does renal agenesis contribute to oligohydramnios? Because less urine is produced leading to oligohyramnios. What is Potter's syndrome? Pulmonary hypoplasia & bilateral renal agenesis. 7. At what week of development are type I and II alveolar cells evident in the developing lung? 26 weeks. What is the earliest developmental period that respiration is possible? Canalicular period. What are type I alveolar cells? Squamous epithelium of endoermal origin. What cells produce surfactant? Type II alveolar pneumocytes. What is surfactant? Complex mixture of phospholipids & two proteins that forms a monomolecular film over the internal walls of the alveolar sacs & counteracts surface T forces at the air-alveolar interface. How is the amniotic fluid removed from the lung at birth? Through the mouth & nose by pressure on the fetal thorax during vaginal delivery, into the pulmonary arteries, veins & capillaries, & into the lymphatics. 8. What is responsible for the occurrence of hyaline membrane disease? Surfactant deficiency. Why is there a protein-rich, fibrin-rich exudate in the alveolar space? It is from a combination of substances in the circulation & from the injured pulmonary epithelium. Who is likely to develop respiratory distress syndrome? Premature newborns. What morphological features of the lung accounts for death in newborns with respiratory distress syndrome? The lungs are underinflated & the alveoli contain the high protein rich fluid which forms a hyaline membrane. 9. How do the lungs of a stillborn and live infant differ? Stillbornfilled with fluid and sink. Live infant filled with air & float. Why do the lungs of stillborn infants sink in water? Because they are filled with fluid. 10. Where do congenital diaphragmatic hernias usually occur and what developmental anomaly is responsible for this condition? They usually occur on the left side of the thorax, so that the heart & mediastinum are usually displaced to the right. It results from defective formation &/or fusion of the pleuroperitoneal membrane with the other 3 parts (septum transversum, dorsal mesentery of esophagus, & muscular ingrowth form lateral body walls) of the diaphragm. This results in a large opening of the posterolateral region of the diaphragm.